Melanoma is infrequent in children, accounting to less than 3% of paediatric cancers, and particularly rare in children aged less than 5 years.1 Due to a low level of suspicion and its clinical similarity with other diseases, its diagnosis is usually delayed. Although its presentation in children is different compared to adults, there are no specific paediatric management guidelines for melanoma.1
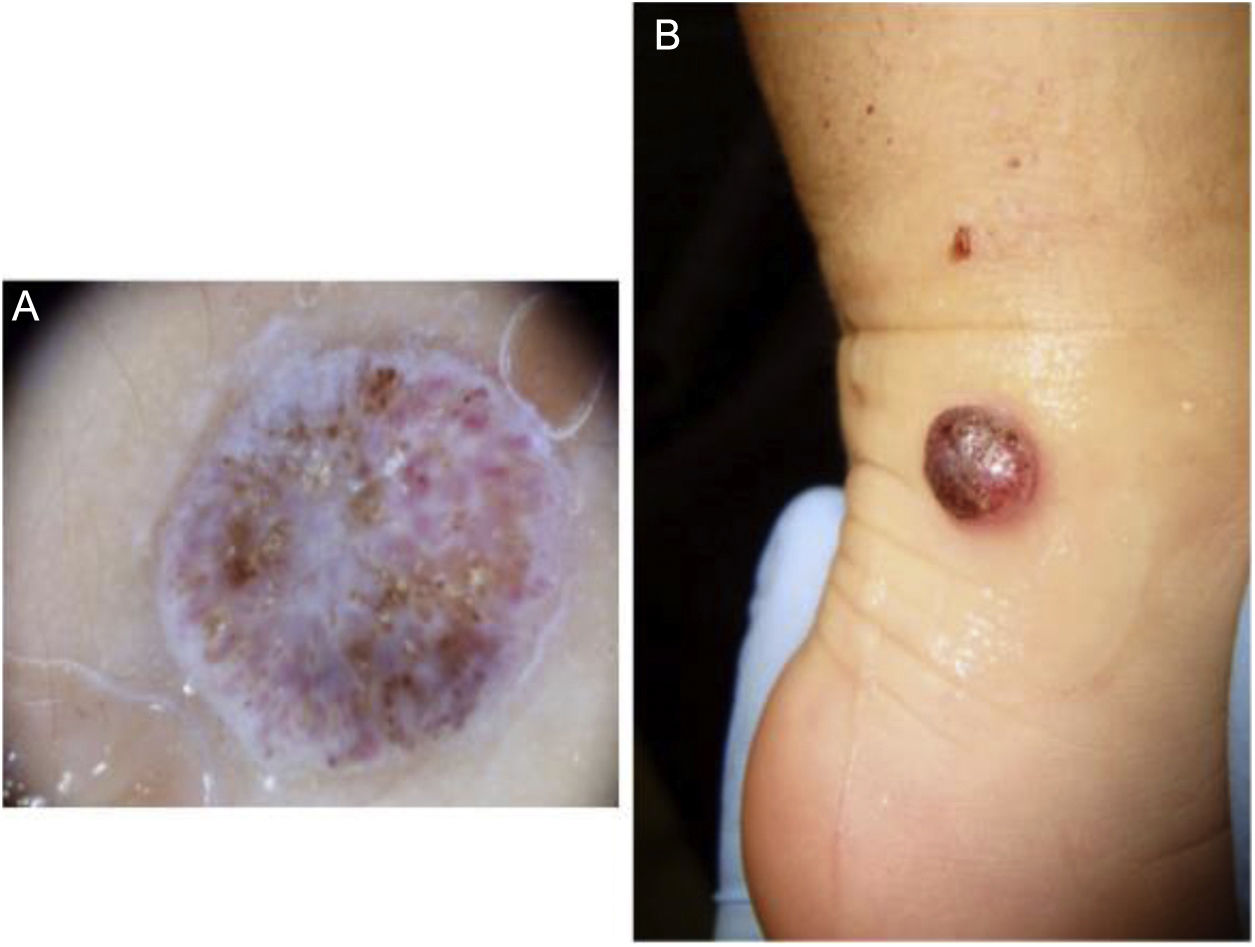
We present the case of female patient aged 12 months with no relevant previous history of disease. She initially presented with a lesion in the right malleolus measuring 0.5 cm in diameter (Fig. 1A), which subsequently progressed to an irregular erythematous dome-shaped nodular lesion (Fig. 1B). In the span of 13 months, the lesion was diagnosed as a traumatic injury, a common wart and telangiectatic granuloma, and the patient underwent a variety of topical treatments, cryotherapy and eventually excision with a biopsy. The histological examination revealed proliferation of atypical fusiform melanocytes with a high mitotic index (>20%). The immunohistochemical assay (Vysis Melanoma FISH Probe kit) revealed overexpression of RREB1 and CNND1 in 80% and 40% of cells, respectively, and the presence of 2 copies per nucleus of Cep6 without amplification of MYB. Eighty percent of cells were Bcl-2 positive. Testing did not detect changes in the BRAF gene (V600E) nor copying number gains in chromosome 11p. The surgical margins, negative, were increased to 4 and 7 mm from the edges due to a Breslow thickness of 2.25 mm, and a sentinel lymph node (SLN) biopsy was performed, revealing subcapsular infiltration. The patient underwent a whole-body positron emission tomography/computed tomography that had negative results. The decision was made to perform a right-side inguinal completion lymph node dissection (negative), with no histological evidence of infiltration in the lymph nodes. The patient received adjuvant therapy with pegylated interferon alfa-2b at a dose of 6 μg/kg/week intravenously for 2 months and 3 μg/kg/week subcutaneously for 11.5 months, which was well tolerated. Ten years later, the patient is still in remission.
Paediatric melanoma is very rare, accounting for less than 1% of diagnosed cases of melanoma, and even less frequent in prepubertal children.2 The differential diagnosis is particularly challenging due to its clinical and histological overlap with other diseases, such as the spectrum of spitzoid tumours and other nonmelanoma skin tumours, requiring participation of an experienced dermatologist and pathologist and molecular testing. Diverging significantly of the characteristic features of melanoma in adults, there are paediatric ABCDE criteria found in less than 40% of cases2: amelanosis, bleeding/ulceration, uniform colour, variable diameter and de novo development. It is typically located in the head, neck or extremities. It usually develops in patients with no pre-existing risk factors.2 There are 3 differentiated subtypes: melanoma arising on congenital nevi, with a significant potential for distant metastasis; conventional melanoma, clinically and molecularly similar to adult-type melanoma; and, as was the case in our patient, spitzoid melanoma, with aggressive locoregional involvement but rarely resulting in distant metastasis.2,3 The prognosis of melanoma at this age is generally excellent. Table 1 summarises the most relevant paediatric melanoma case series.
Review of the most relevant paediatric case series of melanoma in the literature.
| Study | Study period | n | Age (years) | Breslow | Histological subtype | Sentinel lymph node biopsy | Stage | Treatment | Survival | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Brecht et al (2018) | 2002−2012 | 219 | 1−14: 51.6% | <1 mm: 27.8% | SSM: 31.9% | 77% overall | I | Surgery: 100% | 1-year EFS: 93.6% | Same survival in stage III patients that did and did not receive systemic therapy |
| 15−18: 46.6% | 1.01−2 mm: 21.46% | Nodular: 20.5% | 76% in >0.75 mm | : 42.47% | CTX (7) | 3-year EFS: 84% | ||||
| 2.01−4 mm: 19.63% | Spitzoid: 18.7% | (37.5%+) | II: 26.94% | IFN-alfa (19) | 1-year OS: 98% | |||||
| >4 mm: 21.46% | Other: 19.2% | III: 20.09% IV: 4.6% | Immunotherapy (4) | 3-year OS: 91,4% | ||||||
| Undefined: 9.6% | Not specified: 9.65% | NOT REPORTED 5.9% | CTX + IFN alfa (2) | |||||||
| CTX + vemurafenib (1) | ||||||||||
| RT (3) | ||||||||||
| Parikh et al (2017) | 1973−2010 | 917 | <10: 12% | <1 mm: 59% | SSM: 33% | 15.9% (34%+) | I: 69% | RCLND | 10-year OS: no SLNB (99%) vs SLNB (100%) vs SLNB + RCLND (80%) P <.0001 | Sentinel lymph node biopsy did not have an impact on survival |
| 10−19: 88% | 1−2 mm: 17.5% | Nodular: 7% | II: 6.7% | Survival SLNB yes/no: | ||||||
| 2−4 mm: 15% | Malignant, NOS: 52% | III: 15% | Localized 96 vs 98% (P 0.65) | |||||||
| >4 mm: 8% | Desmoplastic: 0.2% | IV: 0.7% | Regional 100 vs 86% (P?) | |||||||
| Other: 7.5% | Metastatic 50 vs 50% P 0.99) | |||||||||
| Richards et al (2016) | 1998−2012 | 84 744 | <18 y: 657 (0.8%) | <18 y vs >18 y | <18 vs >18 y | <18 y vs >18 y | <18 vs >18 y | Immunotherapy: 17.1 vs 3.8% | 5-y OS HR 0.92 vs 0.68 adults | Paediatric patients had more advanced disease at diagnosis, in spite of which they had better outcomes |
| >18 y 98.2% | 1.52 mm vs >0.15 mm | NOS: 56.5 vs 58.7% | 70% (38.5%+) vs 45% (18.5%) | I: 47.7 vs 60.1% | RT: 3.7 vs 4.8% | 10-y OS HR 0.88 vs 0.49 adults | ||||
| Ulceration: 14.7 vs −8.4% | SSM: 24 vs 19.9% | II: 22.9 vs 25.2% | ||||||||
| Nodular: 9 vs 9.8% | III: 28.3 vs 11.8% | |||||||||
| Other: 9.4 vs 1.5% | IV: <1 vs 2.9% | |||||||||
| Mitkov et al (2016) | 2000−2015 | 86 | <17: 50% | 1.2 mm median | SSM: 47.8% | Not documented | I: 51.1% | Not documented | Not analysed | Benign-appearing paediatric skin lesions with a history of evolution, bleeding, or ulceration should raise suspicion for melanoma |
| 17-21 49% | Clark* | Nodular: 8.7% | II: 19.1% | |||||||
| I: 4.8% | Spitzoid: 16.3% | III: 17.1% | ||||||||
| II: 33.7% | Not specified: 27.2% | IV: 12.8% | ||||||||
| III: 18.1% | ||||||||||
| IV: 38.6% | ||||||||||
| V: 4.8% | ||||||||||
| Lorimer et al (2016) | 1998−2011 | 4968 | <10: 6.2% | <10: >2 mm 37% | Not documented | <10: 63% | <10 | <10 | 5-year OS: | No differences in survival based on IFN, SLNB, lymph node dissection. A positive SLNB had no prognostic value in children <10 years. |
| >10: 73.6% | >10: >2 mm 14% | (42%+) | I: 49.6% | IFN: 5.14% | <10 | Greater survival in children vs adolescents | ||||
| 11−20: 51.35% (18.3%+) | II: 13.4% | CTX: 1.61% | SLN−: 94.1% | |||||||
| >20: 44.7% (10.8%+) | III: 37.5% | RT: 1.41% | SLN+: 96% | |||||||
| IV: 17.65% | >10 | >10 | ||||||||
| >10 | IFN: 10.20% | SLN + vs – HR 4.82 (CI 3.38−6.67) | ||||||||
| I: 60% | RT: 0.91% | Cox regression RR | ||||||||
| II: 13.8% | CTX: 2.53% | IFN: 0.77 (0.74−0.80) | ||||||||
| III: 9.3% | RT: 2.60 (2.28−2.96 | |||||||||
| IV: 4.51% | CTX 1.33 (1.25−1.41) | |||||||||
| Kim et al (2016) | 2004−2011 | 310 | <20: 100% | Median: 1.5 mm | Not documented | 84% (28%+) | III: 23% | IFN > EFS | SLN +: 7-year OS 88 vs 66% adults | SLNB is useful for risk stratification, but positive-node status is not associated with a decreased overall survival in children. |
| REST NOT REPORTED | Not analysed as variable involved in OS | Greater survival in paediatric patients compared to adults with the same stage of disease. | ||||||||
| Brecht et al (2015) | 1983−2011 | 443 | <18: 80.7% | <1 mm: 60% | Superficial: 4% | 16% | I-II: 93% | Wide local excision | 5-year OS: 94.8% | Poorer survival with greater depth, nodular melanoma and more advanced disease. |
| <10: 9.3% | >2 mm: 16.5% | Nodular: 1% | (24%+) | III: 5.7% | RCLND | Ulceration: 65.6 vs 99.2% | ||||
| Unknown: 5% | IV: 0.7% | Superficial (100%) vs nodular (77.9%) vs other (96.9%) | ||||||||
| Clark level (I, II, III) 99.1% vs (IV, V) 87.1% | ||||||||||
| Breslow <1 mm (99.1%) vs 1−2 mm (91.4%) vs >2 mm (84.9%) | ||||||||||
| Stage I (98.5%), II (91.1%), III/IV (53%). | ||||||||||
| Cordoro et al (2013) | 1984−2009 | 70 | <10: 27% | <10: 3.9 mm | Not classified | 15% (17%+) | I-II: 0% | Wide local excision | OS 85% | Melanoma characteristics in patients <10 years: |
| 10−20: 72% | >10: 2.4 mm (median) | III: 65.4% | RCLND | Breslow >1 mm 20% | Greater thickness, amelanosis, bleeding, aggressive | |||||
| IV: 34.6% | IFN | Clark | ||||||||
| Clark >III 40% | ||||||||||
| Metastasis: 0% | ||||||||||
| RCLND: 50% | ||||||||||
| IFN: 50% | ||||||||||
| Stage Ib 90% | ||||||||||
| Stage Iib 80% | ||||||||||
| Stage IV 60% | ||||||||||
| Averbook et al (2012) | 1973−2001 | 365 | <10: 6.8% | <10: 2.66 mm | Not analysed | 36.6% (25.4%+) | <10 | Not documented | 10-year OS <10: 100% | Similar effect of depth, ulceration, lymph node involvement and stage on melanoma prognosis in paediatric patients and adults. |
| >10: 93.2% | >10: 1.59 mm | I: 25% | 10-year OS >10: 74.6% | Greater survival in patients <10 years | ||||||
| II: 29.2% | 10-year OS: | |||||||||
| III: 41.7% | Stage I 94.1% | |||||||||
| IV: 4.2% | Stage II 79.6% | |||||||||
| >10: | Stage III: 77.1% | |||||||||
| I: 56.6% | Stage IV: not applicable | |||||||||
| II: 20.7% | 10-year OS GP: 65.5% | |||||||||
| III: 22.1% | 10-year OS ulceration yes/no: 74.1 vs 91.6% | |||||||||
| IV: 2.5% | ||||||||||
| Moore-Olufemi et al (2011) | 1992−2006 | 109 | <10: 23% | <10: >2 mm: 57% | Spitzoid: | <10: 76% (53% +) | <10: | Wide local excision | OS: 89%, no differences between prepubertal children and adolescents | Prepubertal patients had greater Breslow and more frequent SLN involvement. |
| 10-18: 77% | >10: >2 mm: 23% | <10 12% >10 5% | >10: 52% (34% +) | I: 35% | EFS: 73%. 88% in SLN + patients <10 y and 54% for patients >10 y | Higher incidence of paediatric melanoma in patients of Hispanic/Latino ancestry. | ||||
| No Spitzoid: | II: 9% | SLN + status and greater Breslow depth inversely correlated with OS and EFS | ||||||||
| <10 56% >10 84% | III-IV: 57% | |||||||||
| Mixed: | >10: | |||||||||
| <10 20% >10 6% | I: 56% | |||||||||
| Atypical: | II: 21% | |||||||||
| <10 12% >10 y 6% | III-IV: 23% | |||||||||
| Austin et al (2013) | 1998–2007 | 1447 | <10: 11% | <10: | Superficial: 46% | <10: 8.7% | I-II: 73.2% | Not documented | Not documented | Paediatric patients had greater Breslow thickness and more frequent SLN involvement |
| >10−18 y: 89% | ≤1 mm: 21% | Nodular: 5% | (12.6% +) | III-IV: 26.8% | ||||||
| 1.01−2.0 mm: 4% | Desmoplastic: 3% | III: 21% | ||||||||
| 2.01−4.0 mm: 7% | Not specified: 46% | IV: 5.8% | ||||||||
| 4.0 mm: 6% | ||||||||||
| 10−18: | ||||||||||
| ≤1 mm: 39% | ||||||||||
| 1.01−2.0 mm: 6% | ||||||||||
| 2.01−4.0 mm: 5% | ||||||||||
| 4.0 mm: 3% |
CI, confidence interval; RCLND, regional completion lymph node dissection; CTX, chemotherapy; EFS, event-free survival; HR, hazard ratio; IFN, interferon; NOS, not otherwise specified; OS, overall survival; RT, radiation therapy; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; SSM, superficial spreading melanoma; y, years.
Brecht IB, De Paoli A, Bisogno G, Orbach D, Schneider DT, Leiter U, et al Pediatric patients with cutaneous melanoma: A European study. Pediatr Blood Cancer. 2018;65:1−8.
Parikh PP, Tashiro J, Rubio GA, Sola JE, Neville HL, Hogan AR, et al Incidence and outcomes of pediatric extremity melanoma: A propensity score matched SEER study. J Pediatr Surg [Internet]. 2018;53:1753−60.
Richards MK, Czechowicz J, Goldin AB, Gow KW, Doski J, Goldfarb M, et al Survival and surgical outcomes for pediatric head and neck melanoma. JAMA Otolaryngol - Head Neck Surg. 2017;143:34−40.
Mitkov M, Chrest M, Diehl NN, Heckman MG, Tollefson M, Jambusaria-Pahlajani A. Pediatric melanomas often mimic benign skin lesions: A retrospective study. J Am Acad Dermatol [Internet]. 2016;75:706−11.e4.
Lorimer PD, White RL, Walsh K, Han Y, Kirks RC, Symanowski J, et al Pediatric and Adolescent Melanoma: A National Cancer Data Base Update. Ann Surg Oncol. 2016;23:4058−66.
Kim J, Sun Z, Gulack BC, Adam MA, Mosca PJ, Rice HE, et al Sentinel lymph node biopsy is a prognostic measure in pediatric melanoma. J Pediatr Surg [Internet]. 2016;51:986−90.
Brecht IB, Garbe C, Gefeller O, Pfahlberg A, Bauer J, Eigentler TK, et al 443 paediatric cases of malignant melanoma registered with the German Central Malignant Melanoma Registry between 1983 and 2011. Eur J Cancer. 2015;51:861–8.
Cordoro KM, Gupta D, Frieden IJ, McCalmont T, Kashani-Sabet M. Pediatric melanoma: Results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol [Internet]. 2013;68:913–25.
Averbook BJ, Lee SJ, Delman KA, Gow KW, Zager JS, Sondak VK, et al Pediatric melanoma: Analysis of an international registry. Cancer. 2013;119:4012–9.
Moore-Olufemi S, Herzog C, Warneke C, Gershenwald JE, Mansfield P, Ross M, et al Outcomes in pediatric melanoma: Comparing prepubertal to adolescent pediatric patients. Ann Surg. 2011;253:1211–5.
Austin MT, Xing Y, Hayes-Jordan AA, Lally KP, Cormier JN. Melanoma incidence rises for children and adolescents: An epidemiologic review of pediatric melanoma in the United States. J Pediatr Surg [Internet]. 2013;48:2207−13.
Immunohistochemical assays like the one used in our study have exhibited a sensitivity of 85%–100% and a specificity of 95% in discriminating between malignant and benign spitzoid lesions. Gains in 6p25 (RREB1) in more than 63% of cells and in 11q13 (CCND1) in more than 38%, as occurred in our patient, are associated with melanoma and not to benign lesions.2,4 Chromosomal rearrangements leading to kinase fusions (ROS1, NTRK1, ALK, BRAF and RET) are present in 50% of melanomas and can be found in other spitzoid neoplasms, as well as changes in HRAS.3,5 The diagnosis is ruled out in the case of the BRAFv600E and NRAS variants, characteristic of conventional melanoma. Overexpression of Bcl-2 has not proven useful for diagnosis.6 Markers of a poor prognosis are yet unknown, and TERT-p changes and homozygous deletion of 9p21 have been detected in spitzoid melanomas with haematogenous dissemination.3
Despite its different presentation and course, there are no specific guidelines for paediatric melanoma. First-line treatment consists of wide local excision with adequate margins. The indication of sentinel lymph node biopsy is under debate, and followup can be conducted by means of ultrasound.2 In case of regional involvement, regional lymph node excision is no longer considered indicated due to the significant associated morbidity in the absence of prognostic value.2 The use of interferon alfa, highly toxic in adults but safer and better tolerated in children, is also controversial.1,2 The presence of specific variants allows targeted treatments, with BRAF being such an example. Kinase inhibitors may be useful for treatment of spitzoid melanoma with kinase fusion.5
Melanomas are exceptional in the paediatric age group, particularly in prepubertal children, who are more likely to develop spitzoid melanoma. Its differential diagnosis is broad, as it can be confused with other nonmelanoma and spitzoid tumours, and therefore requires a high level of suspicion. It exhibits an aggressive locoregional pattern, in spite of which the prognosis is good. Local treatment is based on the excision of the tumour with wide surgical margins. The molecular profile, markers of metastatic potential and role of adjuvant therapy in this disease have yet to be clearly established.
Please cite this article as: Larrosa C, Torrelo A, Madero L, Lassaletta A. Melanoma en niños prepuberales: dificultades diagnósticas y terapéuticas. An Pediatr. 2022;96:352–357.