The management of Helicobacter pylori infection in children is a consistent problem in clinical practice. Over the years, many questions have been raised regarding symptoms associated with the infection, the diagnostic methods and type of treatment. What is most controversial is determining the criteria that enable us to initiate and carry out the study in children.
In the last 10 years, pediatricians have followed the joint ESPGHAN/NASPGHAN guidelines published in 2011 and updated in 2017 in the management of H. pylori in children.
This document aims to unify the study indication criteria as well as the diagnosis and treatment recommendations for H. pylori infection in children and adolescents, so they can be used in both Primary and Hospital care.
El manejo de la infección por Helicobacter pylori en los niños es un dilema permanente en la práctica clínica. A lo largo de los años se han ido creando multitud de interrogantes respecto a los síntomas ligados a la infección, los métodos diagnósticos y los modos de tratamiento, siendo la más controvertida la indicación diagnóstica.
En los últimos 10 años el colectivo pediátrico ha dispuesto de una guía elaborada por expertos de las Sociedades de Gastroenterología Pediátrica de Europa (ESPGHAN) y Estados Unidos (NASPGHAN) publicada en 2011 y actualizada en 2017 que nos ha orientado en el manejo de la infección por H. pylori en la edad pediátrica.
El presente documento pretende unificar los criterios de indicación de estudio así como las pautas de diagnóstico y tratamiento de la infección por H. pylori en los niños y adolescentes para que puedan ser utilizadas tanto en atención primaria como en la clínica hospitalaria.
Helicobacter pylori is a bacterium that causes chronic gastritis in infected individuals. The infection is acquired during childhood, usually through a household contact, and in most cases persists through adulthood.1 The most frequent form of disease in children is chronic gastritis, and complications are rare during childhood.2
Most infected children are asymptomatic. There is no evidence of an association between infection by H. pylori with chronic abdominal pain compatible with functional disorders. However, ulcers associated with H. pylori infection may cause abdominal pain and upper gastrointestinal bleeding, which is the main indication for diagnostic testing for H. pylori infection.3
Diagnosis in children requires the use of invasive methods with endoscopy. Non-invasive assessment methods are reserved to determine whether H. pylori has been eradicated.1
Pharmacological treatment is guided by the results of antimicrobial susceptibility testing of a gastric mucosa biopsy specimen. The first-line treatment is usually triple therapy with a proton pump inhibitor (PPI) and 2 antibiotics, amoxicillin plus clarithromycin or metronidazole, for 14 days.3H. pylori eradication rates in children have decreased substantially with the increase of antimicrobial resistance, especially to macrolides and to a lesser extent to metronidazole.4
In the past 10 years, a guideline developed by experts of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), published in 20111 and updated in 2017,3 has been available to paediatricians and guided the management of infection by H. pylori in the paediatric population.4
The aim of the document presented by our working group is to standardise the indications for assessment and testing and the approach to the diagnosis and treatment of infection by H. pylori in children and adolescents in light of the considerable variability observed in clinical practice and the increase in the prevalence of antimicrobial resistance reported in recent times, for their application both at the primary care level and in hospital-based care.
MethodologyWorking groupThe working group consisted of 13 experts representing 4 scientific societies: the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition, SEGHNP), the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (Spanish Society of Infectious Diseases and Clinical Microbiology, SEIMC), the Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (Spanish Society of Outpatient and Primary Care Paediatrics, SEPEAP) and the Sociedad Española de Pediatría de Atención Primaria (Spanish Society of Primary Care Paediatrics, AEPap). The work was structured in 3 main sections: general aspects, diagnosis and treatment, agreeing by consensus on the topics to consider. Group members were allocated to review these aspects based on their expertise.
Literature searchWe performed a systematic search in the PubMed/MEDLINE database for articles published between July 1, 2016 (date that the joint ESPGHAN/NASPGHAN guidelines were submitted for publication) and December 31 in English or Spanish, using search terms “Helicobacter pylori” or “Helicobacter”, “Child” and “Adolescent”. We only selected articles focused on humans. The co-authors of each of the 3 sections selected the articles in the literature that were relevant for the purpose of addressing the specific questions formulated in their section.
Development of the documentThe reviews produced by each co-author based on the literature search were shaped into a series of statements, principles and recommendations that were subsequently debated and agreed on by consensus by the entire working group. The full document can be found in the websites of each of the scientific societies that participated in its development.
Results and recommendationsAssessing for H. pyloriP1. When is assessment for H. pylori indicated?The symptoms of infection by H. pylori are nonspecific, and the evidence to date has not established an association between this infection and upper gastrointestinal symptoms in children. Clinicians must investigate the cause of these symptoms by means of upper endoscopy as opposed to only testing for H. pylori. The main indication to test for H. pylori in children with gastrointestinal symptoms is the finding of gastric or duodenal ulcers or erosions on endoscopy, although these features are only found in 5% of the total cases of infection.3,4
Is testing for H. pylori indicated in patients with endoscopic findings other than ulcers or erosions?In children, infection by H. pylori causes chronic inflammation predominantly in the antrum, which usually exhibits a nodular appearance on endoscopy. The presence of antral nodularity in the absence of ulcers does not entail risk to the paediatric patient if H. pylori is not treated and eradicated, but culture of a gastric mucosa specimen should be performed in case the patient undergoes eradication treatment in the future to avoid empirical therapy.3
Is testing for H. pylori indicated in patients with chronic dyspepsia and/or abdominal pain?Chronic or recurrent abdominal pain is one of the most frequent presenting complaints in paediatric care. In most cases, abdominal pain is indicative of a functional disorder, as noted in the Rome IV criteria for functional gastrointestinal disorders, which are characterised by the absence of any identifiable sign of organic disease (Table 1). The diagnosis of these disorders is usually based in the presence of symptoms rather than ruling out other diseases, and therefore it does not always require performance of diagnostic tests.
Warning signs and symptoms in cases of abdominal pain (indications for upper endoscopy).
| Persistent vomiting |
| Dysphagia, odynophagia |
| Haematemesis, melena |
| Unexplained weight loss |
| Decreased linear growth |
| Fever of unknown origin |
| Arthritis |
| Perianal disease |
| Family history of inflammatory bowel disease, coeliac disease or peptic ulcer |
Since the publication of the most recent ESPGHAN/NASPGHAN guidelines,3 several observational studies in large samples of paediatric patients have assessed the association between chronic abdominal pain and infection by H. pylori and have found no evidence of it.5–7 Therefore, we do not consider that testing is indicated in cases of abdominal pain in the absence of signs or symptoms suggestive of an organic disorder.
Which diseases are associated with infection by H. pylori?There is evidence of an association between H. pylori infection and other diseases, but the association has only been reported consistently for some diseases, while the evidence regarding the rest is inconclusive or indirect (Table 2). With the exception of refractory iron-deficiency anaemia,8 chronic primary immune thrombocytopenia (cPIT)9 or mucosa-associated lymphoid tissue (MALT) lymphoma,10 the presence of another disease is not sufficient indication for testing for H. pylori.
Non-gastrointestinal disorders and their association with H. pylori infection.
| Associated diseases | Diseases not proven to be associated |
|---|---|
| Refractory iron-deficiency anaemia | Delayed growth |
| Chronic primary immune thrombocytopenia (cPIT) | Halitosis |
| Mucosa-associated lymphoid tissue (MALT) lymphoma | Obesity |
| Type 1 diabetes | |
| Coeliac disease | |
| Schönlein-Henoch purpura | |
| Recurrent aphthous stomatitis | |
| Atopy | |
| Otitis media with effusion |
No study to date has evaluated the benefits of H. pylori eradication therapy in asymptomatic children with a family history of gastric or duodenal ulcer or stomach cancer in a first-degree relative. In the case of cancer, the ESPGHAN/NASPGHAN guidelines removed the recommendation for testing in 20173 because the issue rarely arises in clinical paediatric gastroenterology practice.
P2. Is the test and treat strategy indicated in paediatric patients?The test and treat strategy involves delivery of H. pylori eradication therapy based on positive results of a non-invasive test, such as the H. pylori breath test or stool antigen test. This strategy is commonly used in the management of adults with dyspepsia in the absence of warning signs, which achieves a small but significant benefit in symptom control.11 However, since similar studies have not been conducted in children, these results cannot be extrapolated to the paediatric population. This practice has resulted in a significant increase in the prevalence of H. pylori resistant to the antibiotics commonly used for its eradication.12
Based on data from the Pediatric European register for treatment of H. pylori, the prevalence of primary resistance to clarithromycin in countries in Southern Europe is substantially greater than 15%, and therefore the use of this drug for empirical therapy is contraindicated. After first-line treatment failure, the prevalence of secondary resistance exceeds 50%, both for clarithromycin and for metronidazole.4 Considering that the range of antimicrobials that can be used in the paediatric population is limited in children, it is key to achieve eradication with the initial treatment, and therefore the selection of drugs for first-line treatment should be based on the results of antibiotic susceptibility testing.
Table 3 presents the recommendations concerning the diagnostic evaluation of H. pylori infection.
Recommendations for assessment of infection by H. pylori.
| When to test for Helicobacter pylori |
|---|
| Recommendation 1. If the findings of upper endoscopy in a child with gastrointestinal symptoms include gastric or duodenal erosions or ulcers, testing for detection of H. pylori should be performed. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 2. If the findings of upper endoscopy are suggestive of H. pylori, for instance, antral nodularity in absence of ulcers, testing is recommended if eradication therapy is being considered. The decision to treat should be made jointly with the family and the patient. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 3. Testing for H. pylori should not be performed in children presenting with abdominal pain and/or dyspepsia suggestive of a functional disorder. If an organic cause is suspected, an upper endoscopy should be performed to assess the aetiology of the complaint. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 4. Testing for H. pylori and eradication therapy are warranted in patients with cPIT or refractory iron-deficiency anaemia. In patients with low-grade MALT lymphoma, eradication therapy is a must, as in most cases it achieves a cure. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 5. Testing for H. pylori and eradication therapy are not indicated in asymptomatic patients with a family history of gastric or duodenal ulcer or cancer. |
| Vote: 9 agreed, 4 abstained, 0 disagreed. Consensus, 70% |
| Recommendation 6. The test and treat approach is not indicated in paediatric patients |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
The initial diagnosis of H. pylori in children should be based on the findings of upper endoscopy.13–15 This examination allows investigation of the aetiology of the presenting symptoms and a definitive diagnosis, and offers the opportunity to obtain a biopsy sample for culture and antimicrobial susceptibility testing to guide treatment, a histological assessment of the severity of the infection and performance of additional testing, such as the urease or polymerase chain reaction (PCR) tests.16,17
The only patients in who diagnosis of infection and subsequent treatment based on non-invasive tests are acceptable are patients with cPIT or at risk of severe complications of upper endoscopy performed under anaesthesia or sedation.
P4. Which tests are appropriate for diagnosis?The diagnosis should be based on culture of a gastric mucosa biopsy specimen or, when the latter is not available or in the absence of growth, on the identification of H. pylori in the histological examination in addition to a positive result of another invasive test (rapid urease test or PCR).15,16,18,19 The use of PCR techniques that can provide information on the resistance to clarithromycin and/or levofloxacin is preferred over methods that can only detect the presence of the bacterium.20 Likewise, we recommend performance of a Gram-stain test in a biopsy sample to strengthen the diagnosis, an option that is particularly valuable when the culture does not grow. Table X in Appendix B (online Supplemental material) presents the different tests that can be performed on biopsy samples.19,21–27
P5. Which treatments should be suspended before testing for H. pylori?Patients should not take antibiotics for a minimum of 4 weeks before performance of upper endoscopy. Proton pump inhibitors must be suspended at least 2 weeks before collection of biopsy samples.3,11,24 If the patient cannot discontinue treatment due to the persistence of symptoms, the PPI can be switched to an H2 antagonist, to be suspended 48h before the test. Ranitidine tablets are no longer commercially available, but hospital pharmacies continue to carry the active ingredient and can make capsules with the amount prescribed by the paediatrician (we recommend a dose of 2mg/kg/12h) or solutions of different concentrations.
P6. How should biopsy samples be collected to guide the diagnosis of infection by H. pylori?A minimum of 6 biopsy samples of gastric mucosa should be collected: 2 each from the antrum and the corpus for pathology, and 1 each from the antrum and the corpus for culture.3 However, we recommend collection of at least one other antrum specimen for performance of additional tests (rapid urease or PCR).
When it comes to sample collection, given the difficulty of growing H. pylori in culture, it is important to take the biopsies before advancing to the duodenum, collecting the samples for culture first with a clean forceps (which should not have been previously inserted in tubes with formalin) and putting them together in a single tube with 1–2mL of normal saline or a specific transport medium. Samples collected for pathology should be placed in tubes with formalin, separating antrum and corpus specimens.19,21,28,29
P7. How should biopsy specimens be stored and transported to the microbiology laboratory to maximize the diagnostic yield?Since H. pylori is a fastidious bacterium and dies easily, appropriately enriched transport media have been formulated to maintain the viability of the samples until they are examined in the microbiology laboratory. When such a medium is not available, normal saline can be used to temporarily prevent drying of the biopsy specimen. In addition, cold temperatures extend the viability of samples and inhibit the proliferation of other microbes contaminating the medium, so if the seeding is going to be delayed, biopsy samples should be stored at a temperature of −20°C or lower if seeding is to happen within 24h and at −70°C or in liquid nitrogen in case of longer delay.29
P8. Should antimicrobial susceptibility testing be performed in every sample sent to the microbiology laboratory?Given the fragility of H. pylori, there is a high risk that the bacterium will not grow in culture and failure due to contamination by other bacteria, so we recommend culture and antimicrobial susceptibility testing of all samples submitted to the microbiology department (at least 1 corpus and 1 antrum sample). On the other hand, although rare, there may be cases of colonization by 2 strains with different drug resistance profiles, an additional reason to perform susceptibility testing in every sample.
Table 4 presents the recommendations for diagnosis of H. pylori infection.
Recommendations for diagnosis of infection by H. pylori.
| How to diagnose infection by Helicobacter pylori |
|---|
| Recommendation 7. Diagnosis of infection by H. pylori will always be performed by means of invasive tests, with collection of biopsy samples during endoscopy. Exceptions can only be made in patients with cPIT and a low platelet count and patients at high risk of complications of procedures performed under sedation or anaesthesia. |
| Vote: 11 agreed, 2 abstained, 0 disagreed. Consensus, 85% |
| Recommendation 8. The diagnosis will be based on the examination of cultures of gastric biopsy samples or, when the latter are not available or the bacterium does not grow in culture, based on the detection of H. pylori in the histological examination in addition to another positive invasive test (rapid urease test or PCR). |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 9. Antibiotic treatment should be suspended at least 4 weeks before the endoscopy. Proton pump inhibitors should be suspended at least 2 weeks before. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 10. We recommend collection of 7 gastric biopsy samples: 2 from the antrum and 2 from the corpus for histological examination, 1 from the antrum and 1 from the corpus for culture, and 1 additional sample to perform other tests (rapid urease test or PCR). The samples for culture should be obtained first and before the endoscope advances into the duodenum. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 11. We recommend transport of biopsy samples in specific transport medium or, if not available, other bacterial transport medium, stored in a refrigerator if the sample is going to be processed within 24h of collection. If transport media are not available, samples can be kept in 1–2mL of normal saline if they can be brought to the microbiology laboratory within 1h of collection. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 12. If samples are going to be submitted to an external laboratory, they must be stored at a temperature of −20°C or lower if testing is not going to be delayed more than 24h or at −70°C or in liquid nitrogen if the delay is going to be longer. |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 13. We recommend performance of culture and antimicrobial susceptibility testing in every biopsy specimen submitted to the microbiology laboratory |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
The first-line treatment for H. pylori eradication in paediatric patients is triple therapy combining one PPI and two antibiotics (amoxicillin plus clarithromycin or metronidazole) given in 2 daily doses for 14 days. The selection of antibiotics is based on the results of susceptibility testing (Table 5), although in Spain the use of metronidazole as opposed to clarithromycin is recommended unless there is evidence of resistance to metronidazole. The dose should be adjusted based on the weight of the patient, and a high dose of the PPI is recommended to achieve adequate acid suppression (Table 6). The aim is to achieve a high eradication rate (>90%) to prevent the development of secondary antibiotic resistances and reduce the need of salvage therapy.3,11
First-line treatment in case of positive culture based on antimicrobial susceptibility.
| HP antimicrobial susceptibility | Recommended treatment |
|---|---|
| Susceptible to CLA and MET | PPI-AMO-MET 14 days at standard dose |
| PPI-AMO-CLA 14 days at standard dose | |
| Sequential therapya | |
| Resistant to CLA, susceptible to MET | PPI-AMO-MET 14 días or bismuth saltsb |
| Resistant to MET, susceptible to CLA | PPI-AMO-CLA 14 days or bismuth saltsb |
| Resistant to CLA and MET or unknown | PPI-AMO-MET 14 days with high-dose amoxicillin or bismuth salts -PPI-AMO-MET |
AMO, amoxicillin; CLA, clarithromycin; MET, metronidazole; PPI, proton pump inhibitor.
In case of penicillin allergy: if the strain is susceptible to CLA and MET, use triple therapy with the standard dose of MET in place of AMO; if the strain is resistant to CLA, use bismuth salts with tetracyclines instead of AMO in patients aged more than 8 years.
Standard doses.
| Drugs | Standard dose | Maximum daily dose |
|---|---|---|
| PPIa | 2mg/kg/day | 80mg |
| Amoxicillinb | 50mg/kg/day | 2g |
| Clarithromycin | 20mg/kg/day | 1g |
| Metronidazole | 20mg/kg/day | 1.5g |
| Bismuth saltsc | 8mg/kg/day | <10 years: 240mg |
| >10 years: 480mg |
All drugs given in 2 doses/day: the PPI 15–20min before meals, and antimicrobial agents during or after breakfast and dinner.
Esomeprazole and rabeprazole are less susceptible than omeprazole to degradation by rapid metabolizers with CYP2C19 variants, which are more frequent in the Caucasian population (56%–81%), and therefore their use is preferred when they are available.3
P10. What is the recommended first-line treatment in the absence of antimicrobial susceptibility testing?The initial treatment should be adapted to the antimicrobial susceptibility profile of the isolated strain, but in case susceptibility is not known, we recommend the following:
- 1.
Adapting treatment to regional or national antimicrobial resistance trends and prescribing the eradication regimen that has proven most effective at the local level.3,14,30
- 2.
First-line triple therapy should not include clarithromycin due to the high prevalence of antimicrobial resistance (>15%). Sequential therapy is also not recommended.3,11,31,32
- 3.
The 2017 ESPGHAN/NASPGHAN3 guidelines concluded that more studies of greater quality were needed to support the recommendation of adjunctive probiotic supplementation during H. pylori eradication therapy.
In our search of the literature published in the past 5 years, we found several studies and systematic reviews with meta-analyses with contradictory results on the efficacy of different strains and combinations of probiotics used as adjunctive therapy during H. pylori eradication treatment. Some studies have found beneficial effects in the prevention of adverse events associated with antibiotic treatment.10,33–35
P12. When and how should the efficacy of eradication therapy be assessed?The effectiveness of eradication therapy must always be assessed.3,13–15 To this end, we recommend the use of validated non-invasive tests: stool antigen test (monoclonal antibody-based enzyme immunoassay) or the 13-C urea breath test.13 In children aged less than 6 years, the stool test is preferable due to the higher frequency of false positives with the breath test and the practical difficulties in performing that test in younger children (Appendix B, Table Y, online Supplemental material).
P13. Which salvage therapy is recommended in case of failure of first-line treatment?The 2017 ESPGHAN/NASPGHAN guidelines3 state that the selection of second-line treatment should be guided by antimicrobial susceptibility testing results or, when these are not available, salvage therapy should include antibiotics not used for first-line treatment and/or greater doses or longer courses of treatment. The proposed salvage regimens were triple therapy with PPI+amoxicillin (75mg/kg/day)+metronidazole or quadruple therapy, adding bismuth, for a total of 14 days.3,15
If antimicrobial susceptibility is not known at the time of first-line treatment, a new upper endoscopy examination and biopsy should be performed to carry out antimicrobial susceptibility testing and prescribe therapy guided by the results. If susceptibility testing is still not available, the strain should be considered double resistant (Table 5).
Also, the use of esomeprazole or rabeprazole is recommended if they were not included in the first-line regimen.11
P14. What can be done to promote adequate adherence to treatment?It is crucial that the physician explain to the family and the patient, too, if age-appropriate, that the success of treatment hinges on adherence, which is associated with a substantial increase in the rate of eradication, with the latter nearing 90% in case of adequate adherence.13,36 Close monitoring during treatment may help achieve this goal.
There are tools that may help prevent missed doses and promote adherence, such as the patient parent guide for treatment of H. pylori developed by the Helicobacter pylori Working Group of the ESPGHAN, which includes a diary to report drug intake, side effects and special events during treatment. A translation to Spanish of this booklet can be downloaded at <https://www.espghan.org/knowledge-center/education/H-Pylori-Patient-Parent-Guide> (Appendix B, Fig. Z, online Supplemental material).
On the other hand, efforts should be made to minimise the side effects of drugs to the extent possible, which is why the use of probiotics is recommended.
Table 7 presents the recommendations for treatment of infection by H. pylori.
Recommendations for treatment of infection by H. pylori.
| How to treat infection by H. pylori |
|---|
| Recommendation 14. The first-line treatment when the antimicrobial susceptibility profile is known is susceptibility-guided triple therapy for 14 days |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 15. When antimicrobial susceptibility is not known, the first-line treatment should not include clarithromycin, and could consist of triple therapy with metronidazole and high-dose amoxicillin or quadruple therapy with bismuth for 14 days |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 16. Concomitant use of probiotics is recommended during eradication therapy on account of its beneficial effect in the prevention of adverse events associated with the use of broad-spectrum antibiotics. The current evidence does not suffice to recommend the use of a particular probiotic formulation to facilitate H. pylori eradication. |
| Vote: 10 agreed, 2 abstained, 1 disagreed. Consensus, 75% |
| Recommendation 17. The effectiveness of eradication therapy has to be assessed, at least 4 weeks after completing treatment, by means of validated non-invasive tests (breath test or stool antigen test) |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 18. In case of first-line treatment failure, the results of the initial antimicrobial susceptibility test should guide decision-making: |
|
|
|
| If the drug resistance profile is unknown, we recommend performance of an additional endoscopic examination and biopsy and performance of culture to guide treatment, and, should the culture not grow, treating the strain as if it were double-resistant |
| Vote: 13 agreed, 0 abstained, 0 disagreed. Consensus, 100% |
| Recommendation 19. It is key to clearly convey to the family the importance of correct adherence to treatment to achieve eradication and reduce the risk of development of antimicrobial resistance. The use of aids and support measures, such as the drug intake diary developed by the ESPGHAN, the prevention of adverse effects of treatment through the administration of probiotics and close monitoring of the patient can improve the likelihood of successful treatment. |
| Vote: 12 agreed, 1 abstained, 0 disagreed. Consensus, 92% |
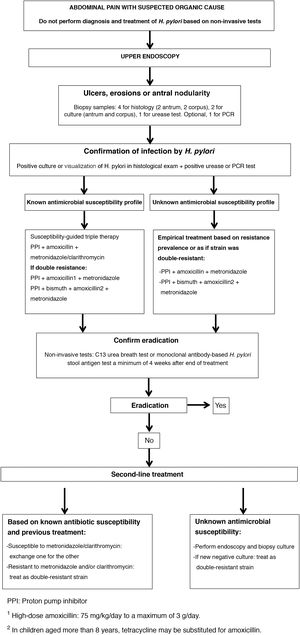
Fig. 1 presents the algorithm for the management of paediatric patients with infection by H. pylori.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Galicia Poblet G, Alarcón Cavero T, Alonso Pérez N, Borrell Martínez B, Botija Arcos G, Cilleruelo Pascual ML, et al. Manejo de la infección por Helicobacter pylori en la edad pediátrica. An Pediatr (Barc). 2021;95:383.