Li-Fraumeni syndrome is a rare cancer predisposition syndrome with an autosomal dominant pattern of inheritance and of variable phenotypic expression associated with germline mutations in gene TP53. This disease predisposes to the development of a wide variety of malignant tumours. The most frequent tumours are soft-tissue sarcomas (rhabdomyosarcoma and others), osteosarcoma, breast cancer in premenopausal women, hypodiploid leukaemia, brain tumours (choroid plexus carcinoma, glioblastoma and medulloblastoma) and adrenocortical carcinoma. These tumours may develop at any age, including in children. The prevalence of this syndrome is not well known, as it is without a doubt underdiagnosed. Since followup of families has not proven to improve long-term survival, to date no programme has been established for detection of affected individuals.
Circumstances are changing in regard to this predisposition syndrome. According to a study published by Villani et al. in 20111 and updated in 2016,2 it is possible to carry out a followup that could potentially increase long-term survival.
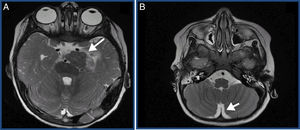
We present the case of a previously healthy boy aged 2 years that visited the emergency services of his local hospital due to a convulsive seizure (complex partial seizure). The evaluation started with imaging tests and, due to the suspicion of a space-occupying lesion, he was transferred to the referral hospital. The imaging tests revealed a malignant tumour with disseminated meningeal involvement throughout the neuraxis, and a primary lesion inside the brain (tumour in the left posterior clinoid region with diffuse meningeal dissemination) (Fig. 1).
(A) Main lesion. Suspected primary tumour. Solid tumour measuring 30mm (CC)×27mm (AP)×20mm (TR) in the left side of the pontine cistern. (B) Disseminated meningeal involvement. Thick irregular enhancement extending through the lateral sulcus and perisylvian cortex, interpeduncular, quadrigeminal and suprasellar cisterns, pineal recess and ventral medulla oblongata.
The histological diagnosis based on the gross and microscopic examination of the biopsy sample obtained by craniotomy was malignant meningioma (high cellularity with cells with a clear cytoplasm and nuclei squeezed to the periphery, other cells with eosinophilic cytoplasm and nuclei pushed to the side, others with large hyperchromatic and pleomorphic nuclei). The cells were arranged in sheets alternating with compressed vessels, with foci of eosinophilic basement membrane material mixed between the tumour cells. Absence of whorls or psammoma bodies. Absence of chondromyxoid stroma. Immunohistochemistry staining: strong positive staining with of CK AE1-AE 3 and INI 1 antibodies. Focal expression of EMA, vimentin, synaptophysin and very focally PLAP. Staining negative for CD 117, OCT3/4 and alpha-fetoprotein. Negative for CD45, CD68, GFAP and desmin. The intense expression of cytokeratins and focal positive staining for EMA and vimentin supported the diagnosis of malignant meningioma and ruled out choroid plexus carcinoma.
The patient was treated with chemotherapy in adherence with the protocol established by the SEHOP for children aged less than 3 years, as surgery and irradiation were not indicated due to the extent of dissemination and the young age of the patient. The patient received the prescribed treatment and complications during followup were managed as they arose (secondary hydrocephalus with a ventriculoperitoneal shunt, convulsive seizures that were difficult to control, refractory vomiting, resting tremor, changes in behaviour). Despite treatment, the disease progressed, and the patient died 6 months after onset.
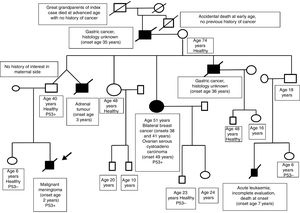
A family history was taken at the time of diagnosis, revealing multiple cases of cancer in the paternal side (see the genetic family tree, Fig. 2). The family was referred to the cancer genetic counselling unit. The diagnosis of Li-Fraumeni syndrome was confirmed by genetic testing (c.430C>T p.Q144* missense mutation in exon 5 of the TP53 of the patient).3 This testing had not been performed before in this family, despite the highly indicative family tree. A subsequent evaluation of the family confirmed that the father and other relatives in his side of the family carried the same mutation.
According to the article published by Villani et al., it is possible to increase overall and long-term survival in carriers of TP53 germline mutations. This article led the American Association for Cancer Research to set up a meeting of international experts on Li Fraumeni syndrome to evaluate and publish the current knowledge on the disease.4 Furthermore, the Li-Fraumeni-Syndrome-Cancer-Predisposition-Syndrome Registry 01 research protocol was launched with their cooperation with the aim of developing a worldwide register of patients with Li-Fraumeni and other cancer predisposition syndromes.
Earlier detection in our patient could have made it possible to modify the course of the disease. Furthermore, other members of the family could have benefitted from adequate followup.5
As paediatricians, it is important that we remain aware of this syndrome when conducting the initial evaluation of a healthy infant in this context. In cases of families with a history of cancer in multiple individuals, the family should be referred to a specialised genetic counselling service.6
The family evaluation would start with an affected adult with the aim of benefitting healthy newborns that could potentially be carriers of the mutation.
Please cite this article as: Gargallo P, Segura V, Yáñez Y, Balaguer J, Cañete A. Li-Fraumeni: ¿la detección de familias aumentaría la supervivencia entre sus miembros? An Pediatr (Barc). 2019;90:54–55.