There are clinical and sociodemographic factors that have an impact on the comfort of the critically ill paediatric patient. The main aim of this study was to determine the level of discomfort of paediatric patients admitted to different national hospitals, and to analyse its correlation with sociodemographic and clinical variables, analgosedation, and withdrawal syndrome.
MethodsAn observational, analytical, cross-sectional, and multicentre study was conducted in five Spanish hospitals. The level of analgosedation was assessed once per shift over a 24 h period, using a BIS sensor, and pain with scales adapted to paediatric age population. The intensity of withdrawal syndrome was determined using the Withdrawal Assessment Tool (WAT-1) scale once per shift for 3 days. Discomfort level was simultaneously assessed using COMFORT Behaviour Scale-Spanish version (CBS-S).
ResultsA total of 261 critically ill paediatric patients with median age of 1.61 years (IQR = 0.35-6.55) were included. An overall discomfort score of 10.79 ± 3.7 was observed during morning compared to 10.31 ± 3.3 during the night. When comparing analgosedation and non-analgosedation groups, statistical differences were found in both shifts (χ2: 45.48; P = .001). At the same time, an association was observed (P < .001) between low discomfort scores and development of withdrawal syndrome development assessed with WAT-1.
ConclusionsAs there is a percentage of the studied population with discomfort, specific protocols need to be developed, guided by valuated and clinically tested tools, like the COMFORT Behaviour Scale-Spanish version.
Existen factores clínicos o sociodemográficos que pueden tener un impacto en el confort del paciente crítico pediátrico. El objetivo principal fue determinar el grado de disconfort de los pacientes pediátricos ingresados en las UCIPs de diversos hospitales nacionales y analizar su relación con variables sociodemográficas y clínicas, sedoanalgesia y síndrome de abstinencia.
MétodosEstudio observacional, analítico, transversal y multicéntrico en cinco hospitales españoles. Se valoró el grado de analgosedación mediante el sensor BIS y las escalas de dolor adaptadas a la edad pediátrica, una vez por tuno durante 24 horas. El grado de abstinencia se determinó con la WAT-1, una vez por turno durante tres días consecutivos. Además, se valoró simultáneamente el grado de disconfort mediante la COMFORT Behavior Scale-versión española (CBS-ES).
ResultadosSe incluyeron un total de 261 pacientes críticos pediátricos con una mediana de 1,61 años (RIQ = 0,35-6,55). Se objetivaron puntuaciones globales de disconfort de 10,79 ± 3,7 en el turno de mañana versus 10,31 ± 3,3 en el de noche. Se observó asociación estadística al comparar al grupo de pacientes analgosedados y los que no en ambos turnos (X2: 45,48; p = 0,001). A la vez, también se observó una relación estadísticamente significativa entre puntuaciones bajas de disconfort y menor desarrollo de síndrome de abstinencia.
ConclusionesExiste una parte de la población estudiada que padece disconfort, por lo que se hace necesario el desarrollo de protocolos específicos guiados por instrumentos válidos y testados en la práctica clínica, como la COMFORT Behavior Scale-versión española.
Paediatric intensive care units (PICUs) are care settings equipped to deliver complex treatments to severely ill patients. The comprehensive care of patients admitted to the PICU usually involves performance of invasive and aggressive diagnostic and therapeutic procedures. In paediatric patients, this may be a source of fear, pain and stress, with the latter known to have a significant deleterious effect on recovery, given the potential physiological repercussions of prolonged stress in a critically ill child, in addition to causing both physical and psychological discomfort1–3.
Some authors recommend keeping critically ill patients alert to facilitate cooperation and early mobilization as a strategy to minimise drug adverse effects, such as withdrawal syndrome and post-intensive care syndrome4. Nevertheless, sedation and analgesia (SA) are still essential components that must be considered in managing the comfort of intensive care patients5. As Caballero et al described, sedation in critically ill patients entails a combination of measures focused on adequate pain management, prevention of delirium, humanization of care, early mobilization and promotion of nocturnal sleep5–7. The use of analgesics and sedatives in the PICU is not free of complications, especially those resulting from under- or oversedation. One of the most frequent complications is withdrawal syndrome stemming from the abrupt discontinuation or rapid tapering of a SA drug, which increases stress in the patient, complicating the course of disease and management in the PICU. In addition, under- or oversedation of the patient can also increase the duration of mechanical ventilation, generate morbidity and increase health care costs on account of a longer stay. Therefore, an adequate assessment of the required level of SA in paediatric critical patients and implementation of safe and individualised interventions to guarantee comfort are essential practices that merit special attention from all intensive care providers8,9. At present, validated instruments are available to assess pain adapted to the specific age and neurologic condition of the patient, such as the Face, Legs, Activity, Cry, Consolability (FLACC) scale10 or the Behavioral Pain Scale, as are scales to assess the level of sedation, such as the Motor Activity Assessment scale, the Sedation-Agitation Scale, the Richmond Agitation Sedation Scale11, the Ramsay sedation score, the COMFORT scale12 and the validated Spanish version of the COMFORT Behavior Scale (CBS-ES)3.
Although in the PICU setting comfort and discomfort depend almost exclusively on the adequate management of sedation and analgesia, there are other possible clinical and sociodemographic contributing factors. Identifying variables potentially involved in the level of discomfort experienced by patients in the PICU could help the care team to establish individualised protocols for the comprehensive care of critically ill patients, reducing heterogeneity in the experience of discomfort and finetuning the dosage of SA to achieve optimal results and avoid under- or overmedication5,8 as well as withdrawal symptoms. To date, few studies have analysed the association between the level of discomfort of critically ill patients and the different sociodemographic and clinical variables that may contribute to it, which is why we deemed that the study would be justified and relevant.
This article presents the results of phase 2 of the COSAIP multicentre study, the objectives of which were to: (1) determine the level of discomfort experienced by paediatric patients admitted to the PICUs of several hospitals throughout Spain and (2) analyse the association between the level of discomfort and sociodemographic and clinical characteristics, sedation and analgesia and withdrawal syndrome.
Material and methodsStudy design and settingWe conducted a cross-sectional multicentre observational and analytical study in the PICUs of 5 hospitals in Spain between May 2018 and January 2020. The units were in the following hospitals: Hospital Universitario Sant Joan de Déu in Barcelona, Corporació Sanitària Parc Taulí, Hospital Universitario 12 de Octubre and Hospital Universitario La Paz in Madrid and Hospital Universitario Carlos Haya in Malaga.
SampleWe recruited patients aged 7 days to 18 years admitted to participating PICUs through nonprobability consecutive sampling applying the following criteria:
Inclusion criteria:
- 1
Willingness to participate and signed informed consent.
- 2
Patient admitted to a participating PICU for a stay longer than 24 hours.
- 3
Intubated patients that received SA continuously for a minimum of 24 h.
Exclusion criteria:
- 1
Patients aged less than 7 days.
- 2
Terminally ill patients.
- 3
Significant language barrier in communication with patient and/or family.
We selected the following sociodemographic and clinical variables: age (in years), sex, underlying disease, reason for admission to PICU, type of SA drugs, type of ventilation, work shift during which the assessment was performed (day shift [8:00-20:00 h] or night shift [20:01-7:59 h]), assessment of the level of discomfort, assessment of SA, adult that accompanied the child and length of stay in the PICU (in days).
We used the Bispectral Index (BIS) to assess the depth of sedation13. A BIS value of 40 to 60 corresponds to a low probability of oversedation4,14, and we used this range to determine whether the depth of sedation in a paediatric patient was adequate. We also used the following pain assessment scales as appropriate for the age of the critically ill paediatric patient: PAIN (age < 1 month), FLACC (patients aged 1 month to 4 years or unable to self-report pain), Faces pain scale (age > 4 years) and numbered pain scale (age > 8 years). We also used the Withdrawal Assessment Tool-1 (WAT-1), which assesses 11 items at different time intervals; it has a maximum score of 12 points and scores of 3 or greater are indicative of withdrawal syndrome15,16.
Lastly, we assessed the level of discomfort with the CBS-ES, structured in 3 dimensions with 2 factors each: (1) alertness and physical movement, (2) calmness/agitation and respiratory response/crying, and (3) muscle tone and facial tension. Scores of 10 or fewer points in this scale indicate absence of discomfort, scores of 11 to 22 presence of discomfort and scores of 23 or greater severe discomfort3.
Data collectionWe designed an ad hoc data collection form that was approved by the entire research team and included the informed consent form, fields to collect sociodemographic and clinical data and the 4 scales used for pain assessment. Prior to initiation of data collection, all researchers participated in an online training that covered the objectives of the study and the instruments used in it. Subsequently, if a patient met the inclusion criteria, researchers sought verbal and written informed consent from the family. Once the family agreed to participation, the sociodemographic and clinical characteristics of the patient were documented in the ad hoc form.
In patients that did not receive SA through continuous infusion, we assessed the level of discomfort by means of the CBS-ES scale and the level of pain with the scale most appropriate for their age.
In patients that received sedative and analgesic drugs together through a continuous infusion pump for a minimum of 24 hours, we recorded BIS monitor values, the score of the pain assessment scale best suited for the patient’s age and the score of the CBS-ES. The observations took place once per shift (day and night shifts) within 24 hours of including the patient in the study. Bispectral index values were considered valid if the signal quality index (SQI) was 95 or greater.
The potential presence of iatrogenic withdrawal was assessed in patients that had received SA for a minimum of 3 days. These assessments were carried out when patients were no longer receiving SA in continuous infusion, using the WAT-1 scale and documenting the level of discomfort using the CBS-ES at the same time, once per shift for 3 consecutive days.
Statistical analysisWe entered the collected data in a database generated with the software IBM® SPSS, version 23.
We summarised quantitative variables with descriptive statistics (mean and standard deviation [SD] or median and interquartile range [IQR]) and categorical variables as absolute frequency and percentage distributions.
To measure the association between quantitative variables, we used the Spearman correlation coefficient, and to measure the association between a quantitative and an ordinal variable, we used the Goodman and Kruskal gamma statistic. To compare quantitative and categorical variables, we used the t test to compare means or the nonparametric Kruskal-Wallis and Mann-Whitney U tests, as applicable. We assessed the association between two categorical variables with the Pearson chi square test.
We set a confidence interval of 95% and considered p-values of less than 0.001 statistically significant.
Ethical considerationsWe obtained the approval of the clinical research and ethics committees of all participating hospitals. The study adhered to the principles of the Declaration of Helsinki (2009), the Belmont Report and Organic Law 1/1996 on the legal protection of the minor and Basic Law 41/2002 on the autonomy of the patient and the rights and obligations in regard to clinical information and documentation.
Participation was voluntary, which was ensured through the obtention of informed consent from a family member. Data were managed in adherence with Organic Law 15/1999 of December 13 on the protection of personal data, so all the data were confidential and privacy safeguarded by assigning a code to each participant in the study prior to handling of the data.
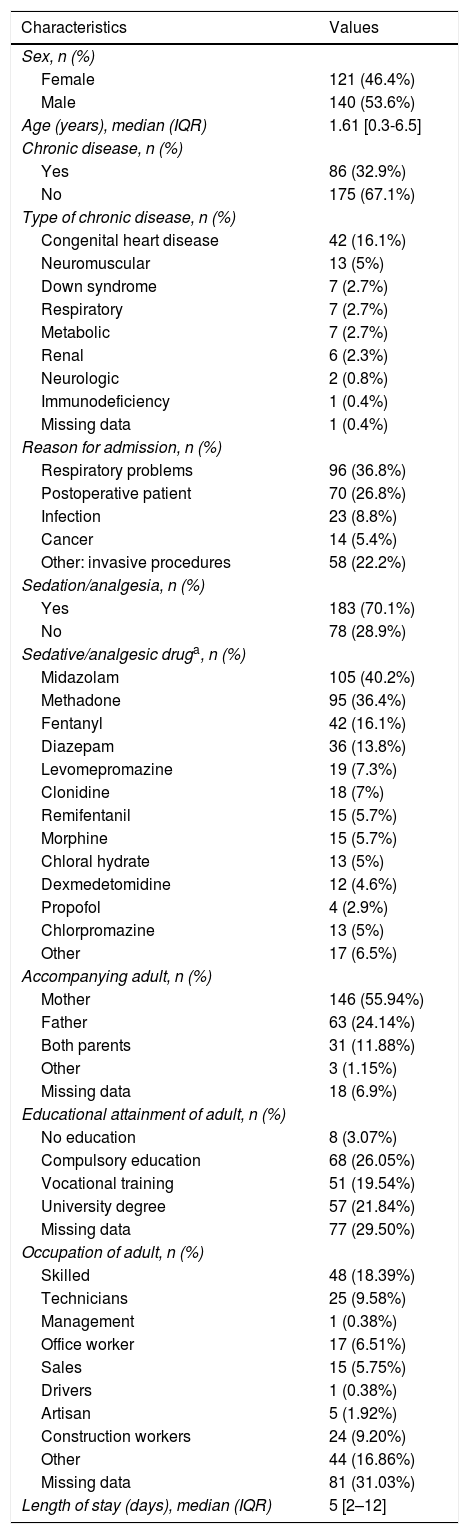
ResultsSociodemographic and clinical characteristics of the sampleThe final sample of the study consisted of 261 patients, 53.64% (n = 140) were male and the median age was 1.61 years (IQR, 0.35-6.55). The most frequent reason for admission to the PICU was respiratory disease, in 36.78% of the patients (n = 96), followed by postoperative care in 26.82% (n = 70). In addition, 32.95% of the patients (n = 86) had underlying chronic disease, most frequently a congenital heart defect (16.09%; n = 42).
When it came to respiratory support, 55.56% required mechanical ventilation, followed in frequency by non-invasive mechanical ventilation (9.96%; n = 26). A total of 70.11% of the patients received continuous SA, and the most frequently used drugs were midazolam (40.23%; n = 105) and methadone (36.40%; n = 95). The median length of stay in the PICU was 5 days (IQR, 2-12) (Table 1).
Sociodemographic and clinical characteristics of the sample (n = 261).
| Characteristics | Values |
|---|---|
| Sex, n (%) | |
| Female | 121 (46.4%) |
| Male | 140 (53.6%) |
| Age (years), median (IQR) | 1.61 [0.3-6.5] |
| Chronic disease, n (%) | |
| Yes | 86 (32.9%) |
| No | 175 (67.1%) |
| Type of chronic disease, n (%) | |
| Congenital heart disease | 42 (16.1%) |
| Neuromuscular | 13 (5%) |
| Down syndrome | 7 (2.7%) |
| Respiratory | 7 (2.7%) |
| Metabolic | 7 (2.7%) |
| Renal | 6 (2.3%) |
| Neurologic | 2 (0.8%) |
| Immunodeficiency | 1 (0.4%) |
| Missing data | 1 (0.4%) |
| Reason for admission, n (%) | |
| Respiratory problems | 96 (36.8%) |
| Postoperative patient | 70 (26.8%) |
| Infection | 23 (8.8%) |
| Cancer | 14 (5.4%) |
| Other: invasive procedures | 58 (22.2%) |
| Sedation/analgesia, n (%) | |
| Yes | 183 (70.1%) |
| No | 78 (28.9%) |
| Sedative/analgesic druga, n (%) | |
| Midazolam | 105 (40.2%) |
| Methadone | 95 (36.4%) |
| Fentanyl | 42 (16.1%) |
| Diazepam | 36 (13.8%) |
| Levomepromazine | 19 (7.3%) |
| Clonidine | 18 (7%) |
| Remifentanil | 15 (5.7%) |
| Morphine | 15 (5.7%) |
| Chloral hydrate | 13 (5%) |
| Dexmedetomidine | 12 (4.6%) |
| Propofol | 4 (2.9%) |
| Chlorpromazine | 13 (5%) |
| Other | 17 (6.5%) |
| Accompanying adult, n (%) | |
| Mother | 146 (55.94%) |
| Father | 63 (24.14%) |
| Both parents | 31 (11.88%) |
| Other | 3 (1.15%) |
| Missing data | 18 (6.9%) |
| Educational attainment of adult, n (%) | |
| No education | 8 (3.07%) |
| Compulsory education | 68 (26.05%) |
| Vocational training | 51 (19.54%) |
| University degree | 57 (21.84%) |
| Missing data | 77 (29.50%) |
| Occupation of adult, n (%) | |
| Skilled | 48 (18.39%) |
| Technicians | 25 (9.58%) |
| Management | 1 (0.38%) |
| Office worker | 17 (6.51%) |
| Sales | 15 (5.75%) |
| Drivers | 1 (0.38%) |
| Artisan | 5 (1.92%) |
| Construction workers | 24 (9.20%) |
| Other | 44 (16.86%) |
| Missing data | 81 (31.03%) |
| Length of stay (days), median (IQR) | 5 [2–12] |
IQR, interquartile range.
A family member accompanied 93.1% of the patients (n = 243), and in 55.94% of cases (n = 146) the mother was the main accompanying adult. The mean age of the accompanying adults was 37.05 ± 7.7 years; 26.05% (n = 68) had completed compulsory education, followed in frequency by 21.84% (n = 57) that had university degrees.
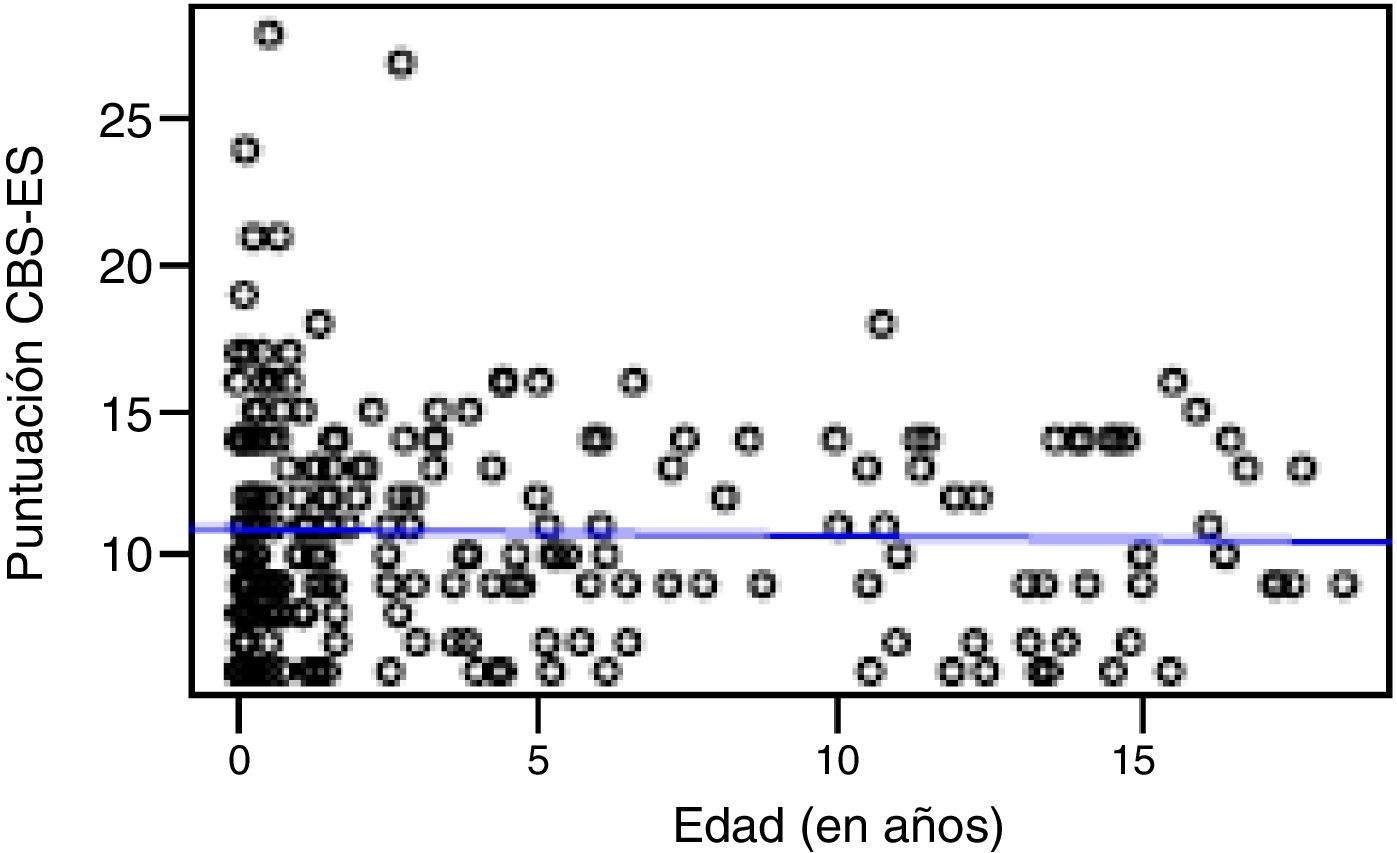
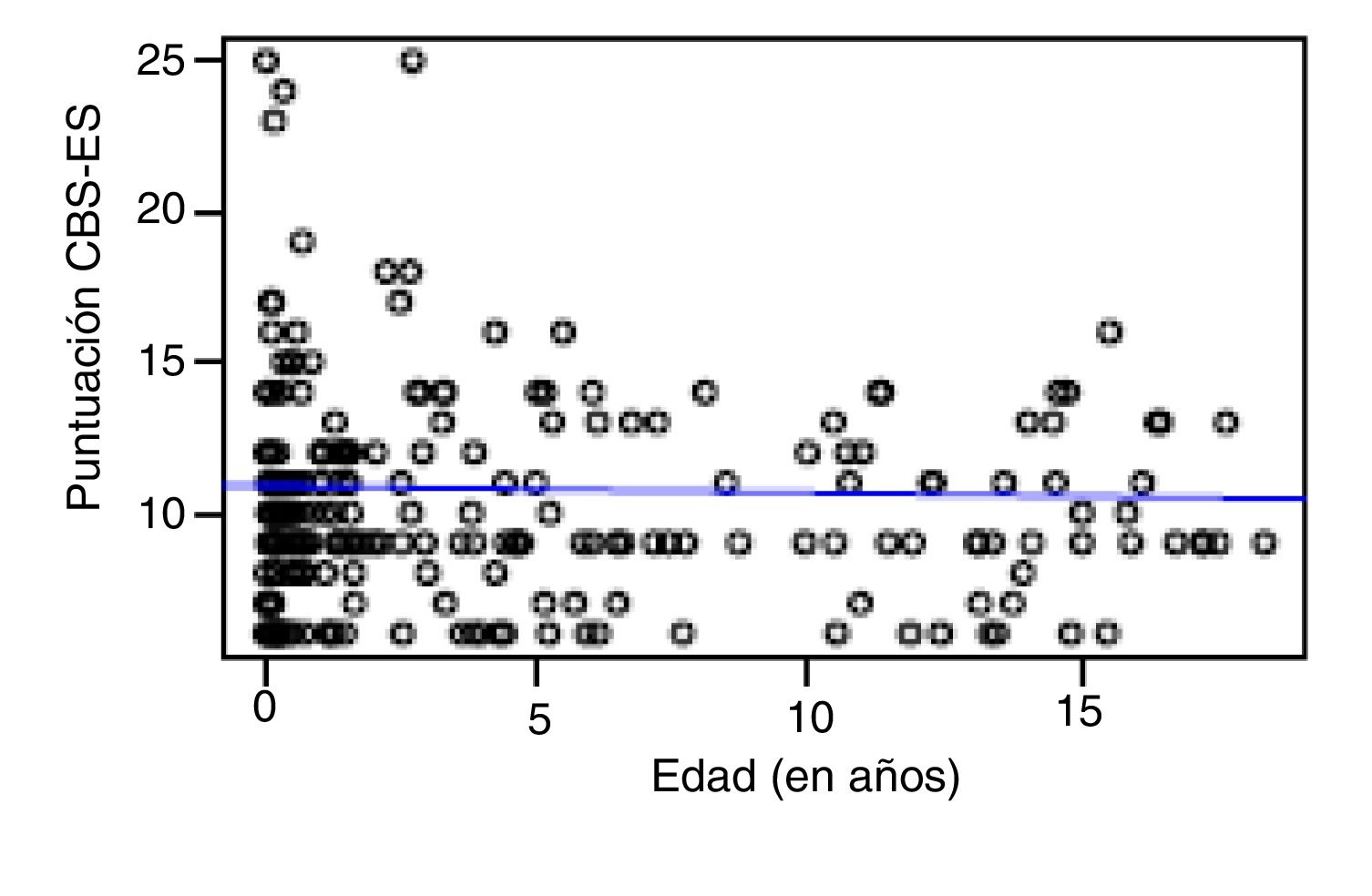
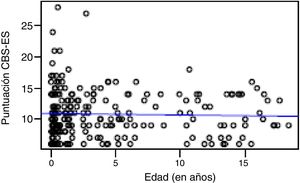
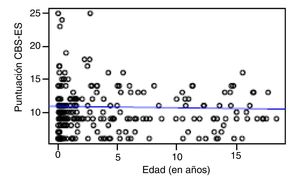
Correlation between the level of discomfort and sociodemographic and clinical variablesIn the total sample, the level of discomfort corresponded to a mean of 10.79 ± 3.7 points in the day shift compared to 10.31 ± 3.3 in the night shift. Sixty-two percent of boys (n = 85) and 61.3% of girls (n = 73) were comfortable (CBS-ES score ≤10 points), without a significant association between the level of discomfort and sex (χ2, 0.01; P = .90). The analysis of the association between the level of discomfort and age in the different shifts (day versus night) did not find statistically significant differences based on age in neither the day shift (rho, 0.03; P = .56) nor the night shift (rho, −0.01; P = .86) (Figs. 1 and 2).
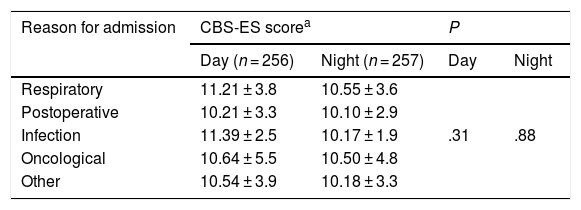
In the day shift, patients admitted with infectious disease exhibited the highest levels of discomfort, with a CBS-ES score of 11.39 ± 2.5, followed by patients with respiratory problems, with a score of 11.21 ± 3.8. In the night shift, it was patients with respiratory problems that had the highest level of discomfort (10.55 ± 3.6 puntos), followed by oncology patients (10.50 ± 4.9 puntos). When we compared CBS-ES scores by reason for admission, we did not find a statistically significant association in neither the day shift (χ2, 4.72; P = .31) nor the night shift (χ2: 1.17; P = .88) (Table 2).
Discomfort scores by reason for admission and work shift (n = 256).
| Reason for admission | CBS-ES scorea | P | ||
|---|---|---|---|---|
| Day (n = 256) | Night (n = 257) | Day | Night | |
| Respiratory | 11.21 ± 3.8 | 10.55 ± 3.6 | ||
| Postoperative | 10.21 ± 3.3 | 10.10 ± 2.9 | ||
| Infection | 11.39 ± 2.5 | 10.17 ± 1.9 | .31 | .88 |
| Oncological | 10.64 ± 5.5 | 10.50 ± 4.8 | ||
| Other | 10.54 ± 3.9 | 10.18 ± 3.3 | ||
CBS-ES: COMFORT Behavior Scale-Spanish version.
The sample included a total of 85 patients with chronic illness in the day shift that had levels of discomfort of 10.61 ± 3.5 points in the CBS-ES scale, compared to 10.88 ± 3.8 points in the group without underlying disease, a difference that was not statistically significant, (χ2, −0.55; P = .58). In the night shift, there were 84 patients with chronic disease with levels of discomfort of 10.65 ± 2.9 points compared to 10.14 ± 3.4 points in patients without underlying disease, a difference that was also not significant (χ2, 3.22; P = .07).
When we analysed discomfort levels in the total sample based on the work shift, although we found lower levels in the night shift (10.31 ± 3.3) compared to the day shift (10.79 ± 3.7), we did not find a statistically significant association between the proportions of patients with and without discomfort and the work shift (χ2, 4.34; P = .03).
Last of all, the mean length of stay in the PICU was 232.85 hours (IQR, 48-300) in patients that experienced discomfort versus 285.52 hours (IQR, 48-336) in patients that were comfortable, and the association between the length of stay in hours and the level of discomfort was not significant (Mann-Whitney U, 2.627; P = .26).
Correlation between the level of discomfort, sedation/analgesia and withdrawal syndromeA total of 183 patients (70.11%) were receiving SA at the time discomfort levels were measured, with a mean BIS of 51.31 ± 15.0 in the day shift and 50.86 ± 15.5 in the night shift. When it came to pain, 93.87% of patients in the day shift and 94.64% in the night shift were pain-free. The mean pain level observed in the total sample was 1.06 ± 0.33 points in the day shift compared to 1.05 ± 0.25 points in the night shift. Seven patients in the day shift (2.68%) had mild pain, followed in frequency by 3 (1.15%) with moderate pain and 1 (0.38%) pain. In the night shift, mild pain was detected in 8 patients (3.07%) and moderate pain in 2 (0.77%).
In the group of patients that received continuous SA, the mean score in the CBS-ES was 9.97 ± 3.8 points in the day shift and 9.79 ± 3.3 in the night shift. In comparison, the corresponding mean scores in the CBS-ES in patients that did not receive continuous SA were 12.65 ± 2.7 and 11.53 ± 2.9. When we compared these groups of patients, we found a statistically significant association between the level of discomfort and the administration of SA in both shifts (χ2: 45.48; P = .001).
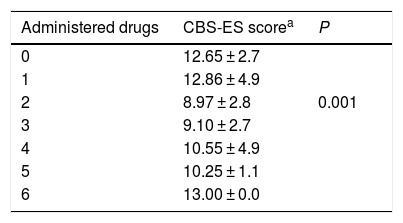
We analysed whether the number of drugs received by critically ill paediatric patients was associated with their level of discomfort, and found that the lowest discomfort scores corresponded to patients receiving 2 drugs (n = 94; 36.7%). This difference was statistically significant ( γ, −0.594; P = .001) (Table 3).
Discomfort score by number of administered drugs
| Administered drugs | CBS-ES scorea | P |
|---|---|---|
| 0 | 12.65 ± 2.7 | |
| 1 | 12.86 ± 4.9 | |
| 2 | 8.97 ± 2.8 | 0.001 |
| 3 | 9.10 ± 2.7 | |
| 4 | 10.55 ± 4.9 | |
| 5 | 10.25 ± 1.1 | |
| 6 | 13.00 ± 0.0 |
CBS-ES: COMFORT Behavior Scale-Spanish version.
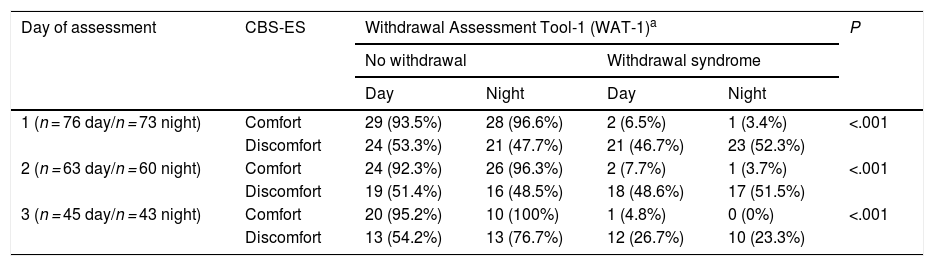
Lastly, when it came to iatrogenic withdrawal, we found an average score in the WAT-1 scale of 1.54 points (0-9) in the day shift and of 1.60 points (0-9) in the night shift. When we made a separate analysis by day of assessment, we found average scores of 1.61 points (0-9) on day 1; 1.65 (0-6) on day 2 and 1.24 (0-6) on day 3 of assessment in the day shift. In the night shift, we found average scores of 1.81 (0-9) on day 1, 1.55 (0-6) on day 2 and 1.26 (0-5) on day 3. As for the discomfort levels measured with the CBS-ES, we found mean scores of 12.96 ± 3.3; 12.73 ± 3.3 and 11.71 ± 2.7 points in the day shift in days 1, 2 and 3, respectively. In the night shift, the scores were 12.77 ± 3.7; 12.37 ± 3.0 and 12.05 ± 2.8 on the same days. When we compared the WAT-1 and CBS-ES scores, we found that the lower the CBS-ES score and therefore the discomfort, the lower the frequency of withdrawal syndrome, an association that was statistically significant in both the day shift (χ2, 14.06; P = .001) and the night shift (χ2, 18.88; P = .001) (Table 4).
Correlation between CBS-ES and WAT-1 scores by day and shift of assessment.
| Day of assessment | CBS-ES | Withdrawal Assessment Tool-1 (WAT-1)a | P | |||
|---|---|---|---|---|---|---|
| No withdrawal | Withdrawal syndrome | |||||
| Day | Night | Day | Night | |||
| 1 (n = 76 day/n = 73 night) | Comfort | 29 (93.5%) | 28 (96.6%) | 2 (6.5%) | 1 (3.4%) | <.001 |
| Discomfort | 24 (53.3%) | 21 (47.7%) | 21 (46.7%) | 23 (52.3%) | ||
| 2 (n = 63 day/n = 60 night) | Comfort | 24 (92.3%) | 26 (96.3%) | 2 (7.7%) | 1 (3.7%) | <.001 |
| Discomfort | 19 (51.4%) | 16 (48.5%) | 18 (48.6%) | 17 (51.5%) | ||
| 3 (n = 45 day/n = 43 night) | Comfort | 20 (95.2%) | 10 (100%) | 1 (4.8%) | 0 (0%) | <.001 |
| Discomfort | 13 (54.2%) | 13 (76.7%) | 12 (26.7%) | 10 (23.3%) | ||
Comfort: score of 6-10 points. Discomfort: score of 11-23 points.
CBS-ES: COMFORT Behavior Scale-Spanish version.
The standardization of the assessment of the depth of sedation and analgesia in critically ill paediatric patients should be one of the priorities of health professionals in the PICU setting13, although the assessment of discomfort is also important. Comfort understood as an outcome resulting from the convergence of several physical and physiological patient-related factors as well as unit-related social and structural factors should be assessed routinely in PICUs throughout the care episode3. The COMFORT Behavior Scale17–21, an instrument that has been adapted for the Spanish population3, may be useful for the purpose.
Our study is one of the first to analyse the level of discomfort in critically ill paediatric patients in a sample of patients admitted to 5 PICUs in Spain. We found that 62% of boys (n = 85) and 61.3% of girls (n = 73) did not experience discomfort, compared to 50.6% of boys (n = 89) and 49.4% of girls (n = 87) in the only similar study found in the literature21. Studies to date have not found an association between the level of discomfort and sex, although one came to the conclusion that sociodemographic factors such as age or sex could have an impact on the perception of pain and discomfort22. Although we did not find published evidence in the paediatrics field that could explain the potential contribution of age to the level of discomfort in this group of patients, a study conducted in the adult population found higher levels of pain and discomfort in young patients23. In addition, among paediatric patients, those who are older are more likely to perceive the intensive care setting as hostile. Thus, while this evidence supports the routine assessment of the level of discomfort in paediatric patients24, future studies should analyse the potential interaction between discomfort and age more in depth, as this could help improve the personalization of the care delivered in the PICU setting.
When we compared CBS-ES scores to the different reasons for admission we did not find statistically significant associations for either the day shift (P = .26) or the night shift (P = .97), which was consistent with the previous study21. The association of discomfort with the presence or absence of chronic disease was also not significant. Differences in dosage in the pharmaceuticals given to either group of patients (with and without chronic disease) could partly explain this, although further research is required to confirm this hypothesis.
The depth of SA is an essential aspect in the management of critically ill paediatric patients25. In our study, this was evinced by the fact that patients who did not receive analgesics or sedatives experienced higher levels of discomfort, a difference that was statistically significant in comparison to patients receiving SA. Several studies provide guidelines for delivery of SA through comfort protocols and using scales such as the COMFORT Behavior Scale or the COMFORT Scale has been shown to reduce the duration of mechanical ventilation, the need of drugs and therefore the incidence of associated adverse events, such as iatrogenic withdrawal, and the length of stay in the PICU1,26–29.
Lastly, on the subject of withdrawal syndrome, we found average scores in the WAT-1 scale of 1.54 (0-9) in the day shift and 1.60 in the night shift (0-9). Therefore, the incidence of withdrawal syndrome was low compared to another study that found iatrogenic withdrawal in 50% of the sample30. Furthermore, the study by Fernández Carrión et al identified midazolam and fentanyl delivered through continuous infusion as the drugs more likely to cause withdrawal syndrome30, a factor that we did not analyse in our study.
When we compared the scores obtained with the WAT-1 and the discomfort scores obtained with the CBS-ES, we found that lower scores in the CBS-ES corresponded to a less frequent detection of withdrawal syndrome, an association that was statistically significant. This suggests that comfort, as defined by the CBS-ES, corresponds to a lower incidence of iatrogenic withdrawal, so the CBS-ES could be a useful instrument to assess for discomfort associated with drug withdrawal and the level of SA. In this regard, we ought to underscore that, as noted in the introduction, the level of discomfort of critically ill paediatric patients may be determined by a variety of factors (environmental factors, work dynamics in critical care units, presence of family members, sociodemographic and clinical variables, etc) which are not all related to the depth of SA. This explains why some patients had scores greater than 10 in the CBS-ES in the analysis of the level of discomfort and withdrawal syndrome.
Lastly, and contrary to expectation, patients that experienced discomfort had shorter lengths of stay in the PICU. A possible explanation is the low mean score in the CBS-ES in patients with discomfort (10.79 ± 3.7 points in the day shift and 10.31 ± 3.3 in the night shift), but further research is required to explore this hypothesis.
LimitationsThe broad age range of the patients, albeit with a predominance of children under 2 years, and the fact that we did not do an analysis stratified by age group, are potential sources of bias when it comes to the association of the level of discomfort and age. Furthermore, we did not take into account which analgesic or sedative gives most comfort to critically ill paediatric patients, or the association between the infusion time and withdrawal syndrome, which are also limitations of our study. This suggests future lines of research to explore which critical paediatric patients experience the most severe discomfort, the effectiveness of specific drugs in alleviating discomfort and the development of adverse events such as iatrogenic withdrawal. Due to the lack of SA scales adapted to the paediatric population and with published psychometric data obtained in the Spanish population, most hospitals assess the depth of sedation by means of the BIS, with the limitations intrinsic to this monitoring system.
ConclusionsOf the patients included in the study, 61.7% were comfortable, but others experienced discomfort. For this reason, one of the most relevant aspects to consider in clinical practice is the ability to identify patients that feel discomfort and treat them so that this proportion can decrease. Based on BIS data and pain scale scores, most children were receiving adequate amounts of SA. In addition, the low scores in the WAT-1 scale indicated correct use of drugs in the clinical management of the paediatric patients under study. Notwithstanding, part of the sample under study did experience discomfort, evincing the need to develop specific protocols guided by valid instruments tested in clinical practice, such as the CBS-ES.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the entire research team for its dedication and help throughout the data collection process and the paediatric patients and families that participated in the study.
Please cite this article as: Bosch-Alcaraz A, Luna-Castaño P, Garcia-Soler P, Tamame-San Antonio M, Falcó-Pegueroles A, Alcolea-Monge S, et al. Grado de disconfort del paciente crítico pediátrico y correlación con variables sociodemográficas y clínicas, analgosedación y síndrome de abstinencia. Estudio multicéntrico COSAIP (Fase 2). An Pediatr (Barc). 2021;95:397–405.