Kawasaki disease (KD) is an acute self-limited systemic vasculitis relatively common in childhood. The etiology of KD is still unknown, although clinical, laboratory and epidemiological features suggest an infectious origin or trigger. Differences on incidence between countries have been related to specific genetic factors, ethnicity, country of birth and some other sociocultural and environmental factors. We present a population-based study on incidence of KD in Catalonia (Spain), focusing on differences between patients in rural and non-rural areas of the region.
MethodsObservational population-based study including all Pediatric Units in Catalan hospitals, between 2004 and 2014. A 12-month (March 2013–March 2014) prospective collection of new cases of KD was carried out to determine the incidence of KD. The rest of the data was retrieved retrospectively.
ResultsData from 399 patients over the 10-year study period was analyzed. Among the total KD patients, 353 (88.5%) lived in non-rural areas and 46 (11.5%) in rural areas. It was found that there is a significant difference (p<0.001) between the percentage of rural population observed in patients with KD (11.5%), and the expected 5% of the Catalan population.
ConclusionThis is the first population-based study showing significant differences on KD incidence rates between rural and non-rural areas.
La enfermedad de Kawasaki (EK) es una vasculitis aguda autolimitada relativamente frecuente en la infancia. La etiología de la EK es aún desconocida, aunque los datos clínicos y de laboratorio y las características epidemiológicas sugieren un origen infeccioso. Las diferencias en la incidencia entre los países se han relacionado con factores genéticos, étnicos y otros factores socioculturales y ambientales. Presentamos un estudio poblacional sobre la incidencia de la EK en Cataluña (España), centrándose en las diferencias entre los pacientes en zonas rurales y no rurales de la región.
MétodosEstudio observacional poblacional incluyendo todas las unidades pediátricas en los hospitales catalanes, entre 2004 y 2014. Recogida prospectiva de nuevos casos de EK durante 12 meses (marzo de 2013-marzo de 2014) para determinar la incidencia de la EK. El resto de los datos se recuperaron de forma retrospectiva. Se analizaron los datos de 399 pacientes durante el período de estudio de 10 años.
ResultadosEntre el total de pacientes con EK, 353 (88,5%) vivían en zonas no rurales y 46 (11,5%) en zonas rurales. Se encontró una diferencia significativa (p<0,001) entre el porcentaje de la población rural observada en los pacientes con EK (11,5%), y el esperado 5% para la población catalana.
ConclusiónEste es el primer estudio poblacional que muestra diferencias significativas entre las tasas de incidencia de EK entre las zonas rurales y no rurales.
Kawasaki disease (KD) is an acute self-limited systemic vasculitis of unknown etiology described by Tomisaku Kawasaki in 1967. It affects predominantly toddlers and children under 5 years old and is the second most common childhood vasculitis in frequency after IgA vasculitis.
KD is a self-limited inflammatory process, but potentially life threatening depending on the extent of cardiac involvement. Diagnosis is based on clinical criteria that include fever, exanthema, conjunctivitis, changes in the extremities, erythema of oral mucosa and lips and cervical lymphadenopathy. An early recognition of the disease is important in order to establish early treatment and to lower the risk of cardiac complications.
The etiology of KD is still unknown, although clinical, laboratory and epidemiological features suggest an infectious origin or trigger.1 However, many studies so far failed to identify a unique etiological infectious agent and the disease has not been proved either to be related to exposure to any drug or to occur as a response to a superantigen.1,2 On the contrary, activation of the immune system is an evident characteristic of KD, and concentrations of many proinflammatory cytokines and chemokines are being studied in patients with KD, which may lead to improved anti-inflammatory therapy in the future.3,4 Due – among other patterns – to the clustering of cases in space and/or time, a reasonable – but still disputed – hypothesis is that KD may be caused by a trigger (probably an infectious agent) that produces disease or elicits response in only genetically predisposed individuals, particularly Asians.
Recent research5,6 has suggested that the causative agent of KD may be an environmental agent carried on trophospheric winds from agricultural sources, possibly a toxin from a fungus. In Japan, burden of cases is seen to be often linked to wind currents originating in a densely cultivated cereal croplands of northeastern China.
To test for a similar predominance of patients from rural areas in other regions of the world where the disease is present, we present a population-based study on the incidence of KD in Catalonia (Spain), focusing on differences between patients in rural and non-rural areas of the region.
Catalonia is an autonomous region in the north-east of Spain in the north-western Mediterranean area. It covers an area of 32,108km2 with a population of 7.5 million inhabitants in 2015, of which non-Spanish immigrants represent about 19% and about 1 million inhabitants with less than 16 years old. Catalonia has a diverse climate and topography. The most populated areas lying by the coast in Tarragona, Barcelona and Girona. The provinces feature a Mediterranean climate and their economy is based on industry, tourism and the tertiary sector. The inland part (including the Lleida province and the inner part of Barcelona province) show a mostly continental climate and agriculture and livestock economies are predominant. The Pyrenean peaks have an Alpine climate at the highest summits, while the valleys have an oceanic climate. Economy is also based on agriculture although tourism plays an important role.
MethodsData sourceEpidemiological, clinical and analytical data was extracted from an observational population-based study including data from patients from all Pediatric Units in 33 Catalan public and private hospitals. Retrospective data retrieval was performed for 10 years (2004–2013) and a 12-month (March 2013–March 2014) prospective collection of new KD cases was carried out to determine the population incidence of KD.
The approval of the ethics committee of the coordinating center (Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain) and the informed consent of patients or their guardians was obtained before initiating the study.
Case definitionAll patients under 16yo who had been diagnosed with KD in their origin hospitals were included in the study. Complete KD was defined by the presence of ≥5 days of fever and ≥4 the 5 classic criteria for KD. These classic criteria included (1) bilateral non-exudative conjunctival injection; (2) oral mucosal changes, such as erythema of the lips or strawberry tongue; (3) changes of the extremities (edema, erythema and/or desquamation); (4) polymorphous rash and (5) cervical lymphadenopathy. Incomplete KD cases were defined according to the guidelines of the American Heart Association2 as patients with ≥5 days of fever and 2 or 3 classic criteria, but who had coronary aneurisms (CAAs) upon echocardiography. Atypical KD was defined as the disease that, although fulfilling classic criteria has atypical features of the disease, such as renal failure or pulmonary impairment.
Exclusion criteria were: those patients that did not fulfill the KD criteria, those whose total days of fever at the beginning of the disease were not specified and those who, despite having KD diagnosis where admitted in hospital for a second opinion from another autonomous region of Spain. Among the patients included in the prospective phase of the study, those not having the informed consent were excluded.
Epidemiological, clinical and analytical information were collected for all patients. Each collaborating center recorded the presence of CAAs according to criteria established in the guidelines of the American Heart Association2 in most cases.
The annual incidence rates of KD in Catalonia and comparison with rural population and ethnicities were calculated based on census data from the Catalan Statistics Institute (IDESCAT). In this study, rural areas were defined as towns and cities with <2000 inhabitants.7
AnalysisData collection was carried using a standardized questionnaire and a Microsoft Office Access 2007 database. SPSS 19.0 statistical software was used for the statistical analysis (IBM Corp., Armonk, NY).
Data are expressed as mean±standard deviation, median with range or number with percentage as appropriate. Parametric and nonparametric comparative tests for continuous data and χ2 test for categorical data were used to compare variables between groups. p<0.05 was considered statistically significant.
ResultsDuring 2004–2014, a total of 399 KD cases were diagnosed in Catalonia. Data from all 33 hospitals with pediatric units in Catalonia was collected, involving the whole Catalonian territory. Of the total 399 patients, 233 (58.4%) had complete KD, 159 (39.8%) incomplete KD and 7 (1.7%) were considered atypical KD. Mean annual incidence was 8/100,000 children<5yo. CAAs were present in 25 patients (6.3%) and a significant difference (p<0.001) between the percentages of ethnic groups was found, the disease being more common in Asian and North African patients.
Among the total KD patients, 353 (88.5%) lived in non-rural areas and 46 (11.5%) in rural areas. A significant difference (p<0.001) was observed toward the percentage of patients living in rural areas (11.5%) versus the expected 5% for Catalan population according to national census.
Conversely, no differences were found regarding age, ethnic group, sex, KD subtype, presence of classical criteria symptoms at the beginning of the disease, risk of CAAs or day of administration of IVIG first dose between children living in rural and non-rural areas. Baseline characteristics of the patients are shown in Table 1.
Comparison of baseline characteristics between rural and non rural areas patients.
| Rural areas (n=46) | Non rural areas (n=353) | p | |
|---|---|---|---|
| Age at diagnosis (m) | 29.7 | 37.9 | 0.264 |
| No. males, n (%) | 23 (50) | 215 (60.9) | 0.201 |
| Kawasaki disease type, n (%) | |||
| Complete | 28 (60.9) | 205 (58.1) | 0.898 |
| Incomplete | 17 (37) | 142 (40.2) | 0.898 |
| Atypical | 1 (2.1) | 6 (1.7) | 0.898 |
| Classical criteria, n (%) | |||
| Conjunctivitis | 40 (87) | 278 (78.8) | 0.194 |
| Lips and oral changes | |||
| Cracked lips | 34 (73.9) | 228 (64.6) | 0.211 |
| Strawberry tongue | 23 (50) | 199 (56.4) | 0.414 |
| Pharyngitis | 21 (45.7) | 177 (50.1) | 0.567 |
| Changes in extremities | |||
| Edema | 21 (45.7) | 140 (39.7) | 0.436 |
| Erythema | 13 (28.3) | 103 (29.2) | 0.898 |
| Desquamation | 17 (37) | 107 (30.3) | 0.360 |
| Exantema | 43 (93.5) | 293 (83) | 0.067 |
| Lymphadenopathy | 13 (28.3) | 102 (28.2) | 0.929 |
| Coronary aneursyms, n (%) | 5 (10.9%) | 47 (13.3%) | 0.643 |
| IVIG administration >10 day, n (%) | 6 (13.3%) | 48 (12%) | 0.337 |
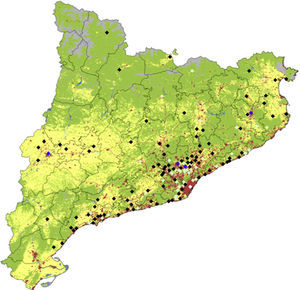
Distribution of cases during the study period, corrected for total population, is shown in Fig. 1. Despite most of the cases being concentrated in the most densely populated areas around Barcelona, there is a significant relatively higher number of cases in regions less densely populated like rural areas of Lleida and Girona.
Catalonia land use map denoting location and number of KD cases. Colors for diamonds are as follows: white: more than 50 cases; blue: 11–25 cases; light green: 2–10 cases and black: 1 case. For land use, green denotes forests, clearings and humid vegetation, gray denotes sand, snow and poor soils, yellow indicates crops and in red, urban and industrial centers (base data for land use obtained from the Generalitat de Catalunya. Department of Environment and Housing).
To the best of our knowledge, this is the first population-based epidemiological study on KD that introduces the possibility of differences in KD incidence between non-rural and rural areas. Among our population, it was found that there is a significant difference (p<0.001) towards the percentage of patients living in rural areas (11.5%) versus the expected 5% for Catalan population according to epidemiological data based on census from IDESCAT.7
It is important to highlight that the study that we present includes KD patients from all over the Catalan territory due to the public health access in our country and the collaboration of all 33 hospitals with pediatric units in Catalonia.
The relationship between other so-called autoimmune diseases and a major incidence in rural areas has also been described in the past. For instance, in primary systemic vasculitis (PSV), a study held in the UK by Lane et al.,8 found that farming during working lifetime was significantly associated with the risk of PSV. Other studies were not able to establish this association in Spain9 or Japan10 and found no differences between incidences on PSV between rural and urban areas. On the contrary, a German study11 found that giant cell arteritis was more prevalent in urban than in rural areas of Germany. In the case of sarcoidosis, some reports suggest that the exposure to bio-aerosols or insecticides could be associated with higher sarcoidosis frequency in the US.12 Another study held in Switzerland13 found association between the regional frequency of sarcoidosis and the presence of vast areas of bread grain and potatoes crops.
The differences between KD incidence in rural and non-rural areas could also be related to the presence of extensive areas of intensive farming,6 sociocultural and household income differences14 or other unidentified environmental or genetic factors that may play an additive role.
A recent study by Rodó et al.6 postulates that a possible wind-borne agent of KD in the form of an environmental toxin could came from the densely cultivated region of north-eastern China. This conclusion was obtained after inferring sources for all days with high KD incidence, obtained by means of daily atmospheric particle backwards simulations. A study performed in the Sichuan province,15 one of the major agricultural production bases of China, found a KD incidence of 7.1/100,000 children, which is lower than incidences described in urban regions of China16,17 or in Japan.18 It remains unclear if this lower incidence is real or if it may reflect less access to medical services in this area. An epidemiologic study performed in Korea between 2006 and 2008,19 found a significant difference on KD incidence according to region, with the highest proportion of cases in the Kangwon province, located in the north-east part of the country, and the lowest on Jeju island, located in the south of the peninsula. Both regions are supported by primary industry (agriculture and fishing). On the contrary, another study about epidemiology of KD in the Netherlands,20 found that the incidence of KD was higher in the provinces with a higher density of population and less agriculture-based economies.
To our knowledge, few other studies describe differences on KD incidence rates between rural and non-rural areas. Inference of air masses provenance at the precise times and locations where the KD kids lived at the times of disease onsets will also be needed to check for consistency with former studies suggesting an agricultural-based etiology. Future larger epidemiological studies are needed to establish the causes of these differences between rural and non-rural areas, to study possible pathogenic etiologies and to establish possible clinical implications for health awareness and policies.
ConclusionsThis is the first population-based study showing significant differences in KD incidence rates between rural and non-rural areas. In this study, KD was found to be more frequent among children living in non-urban areas than expected. Further studies are needed to establish the exact causes of these differences.
FundingNo external funding has been obtained for the realization or publication of the article.
Conflicts of interestThe authors of the article state that they don’t have conflict of interest to disclose. They are not shareholding in a company, they have not received any grant or consultancy fee from a company whose product features in the submitted manuscript or manufactures a competing product.
We would like to thank all the pediatricians from the Kawasaki Disease in Catalonia Working Group for their contribution and Dr. Daniel Cuadras for the statistical support.
Here are the members of the Kawasaki Disease in Catalonia Working Group: Lourdes García (Consorci Sanitari de Mataró), Imma Caubet (Espitau Val d’Aran), Jordi Fàbrega (Fundació Sant Hospital de La Seu d’Urgell), Anna Fernández (Hospital Arnau de Vilanova Lleida), Adolfo Alegre (Hospital de Campdevànol), Isabel Zambudio (Hospital Comarcal Igualada, Consorci Sanitari de l’Anoia), Lluis Delgado (Hospital Comarcal Alt Penedès), Pablo Garcia and Angelita Serrano (Hospital Comarcal Mora d’Ebre), Pere Sala (Hospital de Barcelona), Pilar Villalobos (Hospital de Figueres), Álvaro Diaz Conradi (Hospital de Nens), Joan Agulló (Hospital de Palamós), Socorro Uriz and Aina Sánchez (Consorci Sanitari de Terrassa), Mariona Bonet (Hospital del Mar), Montserrat Gispert-Saüch (Hospital Doctor Josep Trueta Girona), Sonia Corral (Hospital General de Granollers), Zulema Lobato (Althaia Consorci Sanitari de Manresa), Pere Domenech (Hospital General de Vic), Maria Méndez (Hospital Germans Trias i Pujol Badalona), Olga Calavia (Hospital Joan XXIII Tarragona), Marc Tobeña (Hospital MaternoInfantil Vall d’Hebron Barcelona), Emiliano Mora Muñoz (Hospital Mutua de Terrassa), Gemma Sans and Montse Carrera (Hospital Puigcerdà), Ernesto Mónaco (Hospital Sant Camil Sant Pere de Ribes), Anna Ballester (Hospital Sant Jaume de Calella), Anton Foguet (Hospital Sant Jaume Olot), Joaquín Escribano and Neus Rius (Hospital Sant Joan Reus), Angel Moral (Hospital Sant Joan de Déu Martorell), Roser Álvarez (Hospital Sant Pau Barcelona), Toni Sorní (Hospital Verge de la Cinta Tortosa), Vicente Molina (Institut Dexeus Barcelona), Mario Sanz (Parc Sanitari Sant Joan de Déu Sant Boi de Lobregat), Pilar Terradas (Pius Hospital Valls), Salvador Salcedo (Hospital Quirón Barcelona), Olga Martínez (Centre Mèdic Teknon Barcelona).
Please cite this article as: Sánchez-Manubens J, Antón J, Bou R, Iglesias E, Calzada-Hernandez J, Rodó X, et al. En Cataluña la enfermedad de Kawasaki es más prevalente en las zonas rurales. 2017;87:226–231.
The members of the Grupo de Trabajo en Enfermedad de Kawasaki en Cataluña are presented in Appendix A.