Small for gestational age (SGA) newborns show an increased risk of several diseases such as short stature, childhood obesity, Eliminar la comand metabolic comorbidities.
Patients and methodsThe study included 883 patients with obesity (47% females/53% males; mean age: 10.33±3.32 years, BMI: +3.93±1.42 SDS), with prospective follow-up (5 years) of growth, recording adult height when achieved (n=104). Comparisons at diagnosis, according to their neonatal anthropometry; adequate for gestational age (AGA; n=810) vs. SGA (n=73), were performed for the following features: age at their first visit, standardised height for target height (z-score), bone age, adult height prediction, BMI (z-score), glycaemia, insulinaemia, HOMA, total cholesterol, HDL, LDL, triglycerides, 25-OH-vitamin D, area under the curve (AUC) for glucose and insulin in the OGTT, LDL/HDL and triglyceride/HDL ratio, insulin-like growth factor (IGF-I) and IGF-binding protein 3 (IGFBP-3) serum levels.
ResultsDespite similar BMI-SDS, ethnic, and pubertal distribution in both groups, patients with SGA showed more severe changes in lipid profile (triglyceride and triglyceride/HDL ratio, both P<.05) and carbohydrate metabolism (higher glycaemia, glucose and insulin AUCs, HOMA, HbA1c and lower whole-body insulin sensitivity index (WBISI), all P<.05) and lower 25-OH vitamin D levels (P<.05). They also showed a poorer adult height prediction (adjusted for target height) (P<.01), despite a similar degree of advance in skeletal maturation and similar IGF-I and IGFBP-3 levels than AGA patients.
ConclusionsThe background of SGA neonatal anthropometry is associated with a higher prevalence and severity of metabolic comorbidities and to a poorer adult height prediction in obese children and adolescents.
Los recién nacidos pequeños para su edad gestacional (PEG) presentan mayor riesgo de sufrir diversas enfermedades, tales como talla baja, obesidad infantil y sus comorbilidades metabólicas.
Pacientes y métodosEstudio de 883 pacientes con obesidad (47% niñas/53% niños; edad: 10,33±3,32 años, IMC:+3,93±1,42 SDS) con seguimiento prospectivo (5 años) del crecimiento para registro de talla adulta (n=104). Se compararon al diagnóstico según hubiesen presentado antropometría neonatal adecuada (AEG; n=810) o PEG (n=73), las siguientes variables: edad en primera consulta, talla estandarizada (Z-score) respecto a talla diana, edad ósea, predicción de talla adulta, IMC estandarizado (Z-score), glucemia, insulinemia, HOMA, colesterol total, HDL, LDL, triglicéridos, 25-OH-vitamina D, área bajo la curva (AUC) de glucemia/insulinemia en el TTOG, cocientes LDL/HDL y CT/HDL y niveles de IGF-I e IGFBP-3.
ResultadosLos pacientes nacidos PEG presentaban (a igualdad de edad, IMC-SDS, distribución étnica y puberal) una afectación más intensa del metabolismo lipídico (triglicéridos e índice triglicéridos/HDL superiores, ambos p<0,05) e hidrocarbonado (mayores niveles de glucemia, AUC de glucosa e insulina, HOMA, HbA1c y menor WBISI, todos p<0,05), así como menores niveles circulantes de vitamina D (p<0,05). Asimismo, presentaban un peor pronóstico de talla adulta con respecto a su talla diana (p<0,01), pese a mostrar un grado similar de aceleración de la maduración esquelética y niveles comparables de IGF-I e IGFBP-3 que los AEG.
ConclusionesEl antecedente de antropometría neonatal PEG se asocia a una mayor frecuencia e intensidad de alteraciones metabólicas y a un peor pronóstico de talla adulta en los niños y adolescentes obesos.
Childhood obesity is defined as a risk of early morbidity and mortality resulting from the excessive accumulation of adipose tissue.1 Similarly, and as is the case of other chronic diseases, multiple factors that influence the intrauterine environment and the first years of extrauterine life have an impact on obesity. Thus, suboptimal nutrition or undernutrition in utero, nutritional practices in the first months of life and weight gain in early childhood may play a relevant role in the development of obesity and of metabolic disorders in future stages of life.2
The term small for gestational age (SGA) refers to a newborn whose weight or length is 2 or more standard deviations (SDs) below the mean for sex and gestational age in the reference population.3 Since this definition is solely based on neonatal anthropometric measurements, infants born SGA constitute a highly heterogeneous group with a broad range of possible aetiologies (fetal, maternal, placental, etc.).4 While the term encompasses both newborns with low birth weight and newborns with low birth length, it may be useful to differentiate 3 groups among infants born SGA: low birth weight, low length, or both low weight and low length (z-scores).5 This classification could help to guide the aetiological diagnosis and to assess the risk of future comorbidities.
It is estimated that between 2.3% and 10% of children are born SGA.6,7 Most of them exhibit spontaneous catch-up growth by 2 years of age and a small proportion between 2 and 4 years of age, but approximately 15% do not experience this catch-up growth, which is an accepted indication for recombinant human growth hormone (rhGH) replacement therapy.
Among the children born SGA that experience spontaneous catch-up growth, those born with low weight following a period of fetal growth restriction are more likely to develop a higher proportion of body fat content in the first years of life in the context of the rapid weight gain of the catch-up process compared to children that have anthropometric measures appropriate for gestational age at birth.8 This “adiposity rebound”, which occurs earlier compared to its usual timing in childhood,9 involves a higher risk of future obesity and the associated metabolic complications (involving both carbohydrate and lipid metabolism). These findings overlap to a certain extent with the patterns observed in children born preterm, in whom it has been demonstrated that rapid weight gain during early childhood, even in the first few weeks after birth, can lead to hypertension, obesity and related disorders before the third decade of life.10–13
Nutritional imbalances during intrauterine life and in the period following birth can have an impact not only on body composition14 but also on linear growth in the short and the long term,15,16 which may be due to restricted growth during the fetal period, the process of postnatal catch-up growth, or both. Generally, childhood obesity is associated with a more rapid prepubertal growth and a variable degree of advancement in bone maturation.17 However, when it comes to patients with a history of SGA, it would be fair to assume that this growth pattern could be influenced and modified by the potential deleterious effect on linear growth of fetal growth restriction.
To date, many studies have assessed the postnatal growth patterns and the future risk of obesity and associated comorbidities in children born SGA. In comparison, the inverse association has been a much less frequent subject of study, that is, the importance of the anthropometric factor of having a history of SGA in the management of obese children or adolescents, in terms of both the development of metabolic comorbidities and linear growth.
The aim of our study was to analyse the impact over time of a history of SGA (determined based on neonatal anthropometric measurements) in a large cohort of obese children and adolescents on their anthropometric characteristics (height and severity of obesity) and metabolic characteristics, comparing them to patients born with anthropometric measurements appropriate for gestational age (AGA).
Patients and methodsOur study included 883 patients (415 [47%] female and 468 [53%] male) with obesity (BMI>+ 2 SDS for age and sex, using the growth tables of Hernández et al. as reference18) managed at a tertiary care hospital, excluding patients with syndromic or secondary obesity or with underlying disease.
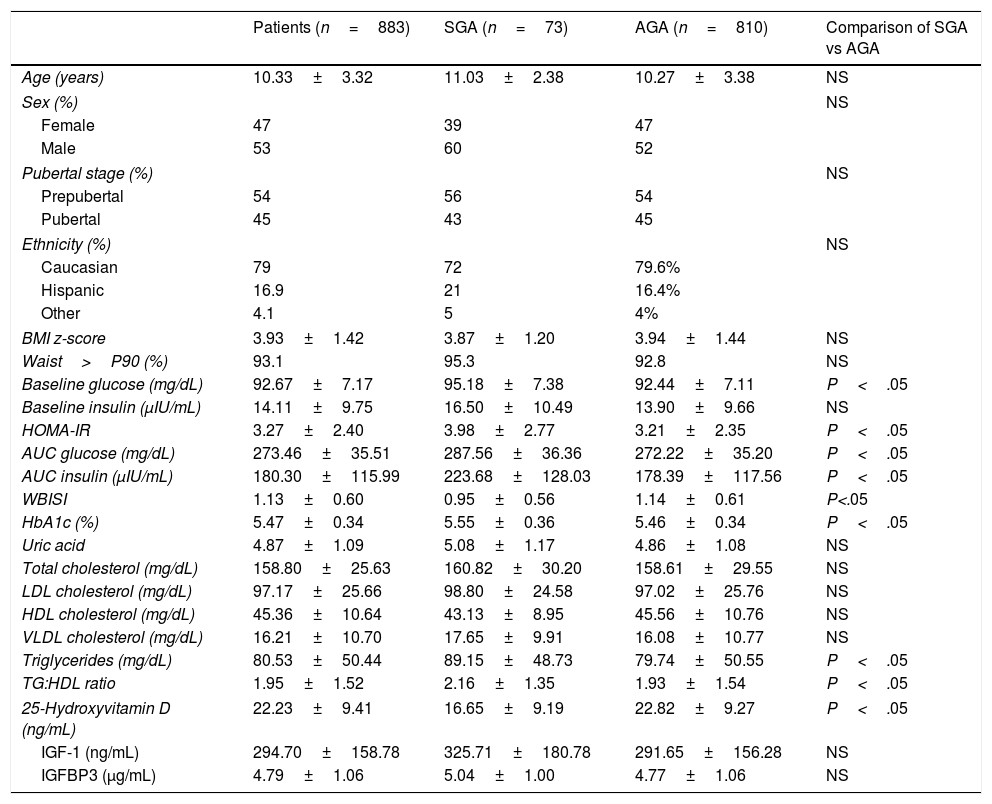
In this cohort, 810 of the patients had neonatal anthropometric measures that were appropriate for gestational age (AGA defined as z-scores for birth weight and length between −2 and + 2) and 73 had been born small for gestational age (SGA defined as z-scores for birth weight or length<−2) using the population data published by Carrascosa et al.19 as reference; these were the 2 groups compared in the study. We excluded patients with syndromic or secondary obesity or with underlying disease from the overall sample, and in the SGA group, we excluded patients that did not exhibit catch-up growth, or with rhGH replacement therapy. On the other hand, we did not exclude patients born preterm (gestational age<37 weeks), which amounted to 8.2% of the SGA group and 7.9% of the AGA group. Table 1 summarises the characteristics of patients in the SGA and AGA groups.
Anthropometric and metabolic characteristics of the cohort under study.
| Patients (n=883) | SGA (n=73) | AGA (n=810) | Comparison of SGA vs AGA | |
|---|---|---|---|---|
| Age (years) | 10.33±3.32 | 11.03±2.38 | 10.27±3.38 | NS |
| Sex (%) | NS | |||
| Female | 47 | 39 | 47 | |
| Male | 53 | 60 | 52 | |
| Pubertal stage (%) | NS | |||
| Prepubertal | 54 | 56 | 54 | |
| Pubertal | 45 | 43 | 45 | |
| Ethnicity (%) | NS | |||
| Caucasian | 79 | 72 | 79.6% | |
| Hispanic | 16.9 | 21 | 16.4% | |
| Other | 4.1 | 5 | 4% | |
| BMI z-score | 3.93±1.42 | 3.87±1.20 | 3.94±1.44 | NS |
| Waist>P90 (%) | 93.1 | 95.3 | 92.8 | NS |
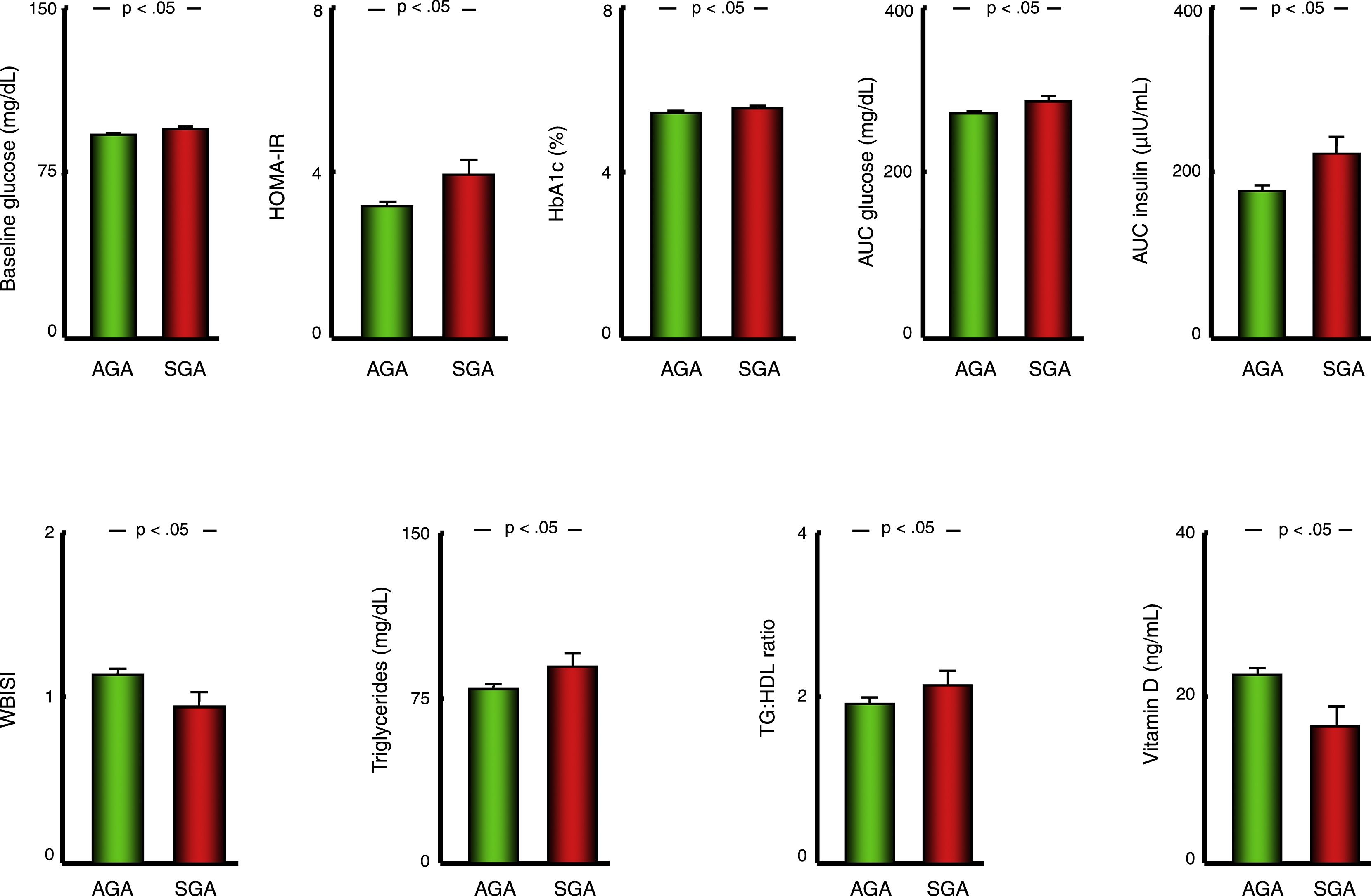
| Baseline glucose (mg/dL) | 92.67±7.17 | 95.18±7.38 | 92.44±7.11 | P<.05 |
| Baseline insulin (μIU/mL) | 14.11±9.75 | 16.50±10.49 | 13.90±9.66 | NS |
| HOMA-IR | 3.27±2.40 | 3.98±2.77 | 3.21±2.35 | P<.05 |
| AUC glucose (mg/dL) | 273.46±35.51 | 287.56±36.36 | 272.22±35.20 | P<.05 |
| AUC insulin (μIU/mL) | 180.30±115.99 | 223.68±128.03 | 178.39±117.56 | P<.05 |
| WBISI | 1.13±0.60 | 0.95±0.56 | 1.14±0.61 | P<.05 |
| HbA1c (%) | 5.47±0.34 | 5.55±0.36 | 5.46±0.34 | P<.05 |
| Uric acid | 4.87±1.09 | 5.08±1.17 | 4.86±1.08 | NS |
| Total cholesterol (mg/dL) | 158.80±25.63 | 160.82±30.20 | 158.61±29.55 | NS |
| LDL cholesterol (mg/dL) | 97.17±25.66 | 98.80±24.58 | 97.02±25.76 | NS |
| HDL cholesterol (mg/dL) | 45.36±10.64 | 43.13±8.95 | 45.56±10.76 | NS |
| VLDL cholesterol (mg/dL) | 16.21±10.70 | 17.65±9.91 | 16.08±10.77 | NS |
| Triglycerides (mg/dL) | 80.53±50.44 | 89.15±48.73 | 79.74±50.55 | P<.05 |
| TG:HDL ratio | 1.95±1.52 | 2.16±1.35 | 1.93±1.54 | P<.05 |
| 25-Hydroxyvitamin D (ng/mL) | 22.23±9.41 | 16.65±9.19 | 22.82±9.27 | P<.05 |
| IGF-1 (ng/mL) | 294.70±158.78 | 325.71±180.78 | 291.65±156.28 | NS |
| IGFBP3 (μg/mL) | 4.79±1.06 | 5.04±1.00 | 4.77±1.06 | NS |
AGA, patients born with anthropometric values appropriate for gestational age; AUC, area under the curve; BMI, body mass index; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; IGF-1, insulin-like growth factor 1; IGFBP3, insulin-like growth factor binding protein 3; LDL, low-density lipoprotein; SGA, patients born with anthropometric values small for gestational age; TG, triglycerides; VLDL, very low-density lipoprotein; WBISI, Whole-Body Insulin Sensitivity Index.
For all patients, we recorded the chronological age at onset of obesity, and in the first related medical visit, recorded the weight, height and BMI (standardized adjusting for age and sex),18 waist circumference and pubertal stage.20 We estimated bone age based on X-rays of the left hand and wrist (Greulich and Pyle [GP] method), predicted the final height (Bailey-Pinneau [BP] method) and compared it to the genetic height potential (modified mid-parental height [+6.5cm for boys and −6.5cm for girls]). We carried out a prospective followup of linear growth over 5 years, recording the final height of those patients that reached it during the study (n=104).
We collected blood samples from patients following a 12-h fast to measure the following serum levels: glucose, insulin (chemiluminescent immunoassay, Liaison® DiaSorin, Saluggia, Italy), glycated haemoglobin (HbA1c) (ion-exchange high performance liquid chromatography, D10TM, Bio-Rad, Hercules, California, United States), total cholesterol, cholesterol fractions (high density lipoprotein [HDL], low density lipoprotein [LDL], and very low density lipoprotein [VLDL]) and triglycerides (enzymatic colorimetric assay, Beckman® AU680, Brea, California, United States), uric acid, 25-hydroxyvitamin D (chemiluminescent immunoassay, Lumipulse G®, Fujirebio Diagnostics AB, Goteborg, Sweden), insulin-like growth factor 1 (IGF-1) and insulin-like growth factor-binding protein 3 (IGFBP-3) (chemiluminescent immunoassay, Liaison® DiaSorin). We also calculated the homeostatic model assessment index of insulin resistance (HOMA-IR: fasting glucose [mg/dL]×fasting insulin [μIU/mL]/405).
An oral glucose tolerance test (OGTT) was performed in 569 patients (45 born SGA and 524 AGA) with administration of 1.75g glucose per kilogram of body weight to a maximum of 75g and measurement of glucose and insulin serum levels at 30, 60 and 120 after ingestion of the glucose. We calculated the receiver operating characteristic (ROC) area under the curve (AUC) of the glucose and insulin levels (AUC: 0.25×baseline value+0.5×30min value+0.75×60min value+0.5×120min value) and the Whole-Body Insulin Sensitivity Index (WBISI: 10000/square root ([glucose×insulin]×[mean glucose in OGTT×mean insulin in OGTT]).21
We performed the statistical analysis of the data using SPSS® version 15.0 for Windows (MapInfo Corporation; Troy, New York, United States). We have expressed the values of the different variables as mean±standard deviation or median±SD for values that were standardised based on data for a reference population. We defined statistical significance as a P-value of less than 0.05. For normally distributed variables, we compared the means of two groups using the independent sample t test (after verifying the normal distribution of the data by means of the Kolmogorov–Smirnov test). When the assumption of normality was not met, we used the Mann–Whitney U test to compare the two groups. We compared nonparametric paired samples by means of the Wilcoxon rank test.
The protocol of the study and of patient recruitment was approved by the Ethics Committee of the hospital as part of a research project funded by the Fondo de Investigación Sanitaria of the Instituto de Salud Carlos III, and adheres to the ethical principles for medical research involving human subjects established in the Declaration of Helsinki by the World Medical Association. We obtained the informed consent of the parents or legal guardians of all participating patient, as well as the assent of participants aged more than 12 years.
ResultsWe did not find significant differences in the distribution of sex, pubertal stages and ethnicity between the SGA and AGA groups, and the mean BMI SDS was similar in both groups (Table 1).
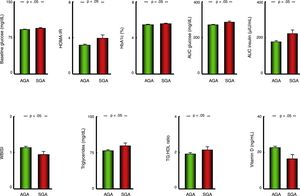
Metabolic comorbiditiesPatients with obesity born SGA had significantly higher serum levels of glucose and HbA1c and HOMA-IR values, and greater AUCs for both glucose and insulin with a lower WBISI in the OGTT compared to patients with obesity born AGA (Fig. 1). Furthermore, patients with anthropometric measures consistent with SGA had higher levels of circulating triglycerides and higher triglyceride-to-HDL cholesterol ratios (Fig. 1) and lower serum vitamin D levels compared to patients born AGA (Table 1).
Bar charts presenting the values of variables used to assess carbohydrate metabolism, the lipid profile and vitamin D status in patients with a history of born AGA or SGA. Data expressed as mean±standard deviation. AGA, patients born with anthropometric values appropriate for gestational age; AUC, area under curve; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; SGA, patients born with anthropometric values small for gestational age; TG, triglycerides; VLDL, very low-density lipoprotein cholesterol; WBISI, Whole-Body Insulin Sensitivity Index.
Applying the criteria for the definition of metabolic syndrome (MS) proposed by the International Diabetes Federation,22 in the subset of patients aged more than 10 years with a waist circumference above the 90th percentile for age, sex and ethnicity, 94 patients met the criteria for MS (10.6%), corresponding to 12 in the SGA group (16.4%) and 82 in the AGA group (10.1%), although this difference in prevalence between groups was not statistically significant.
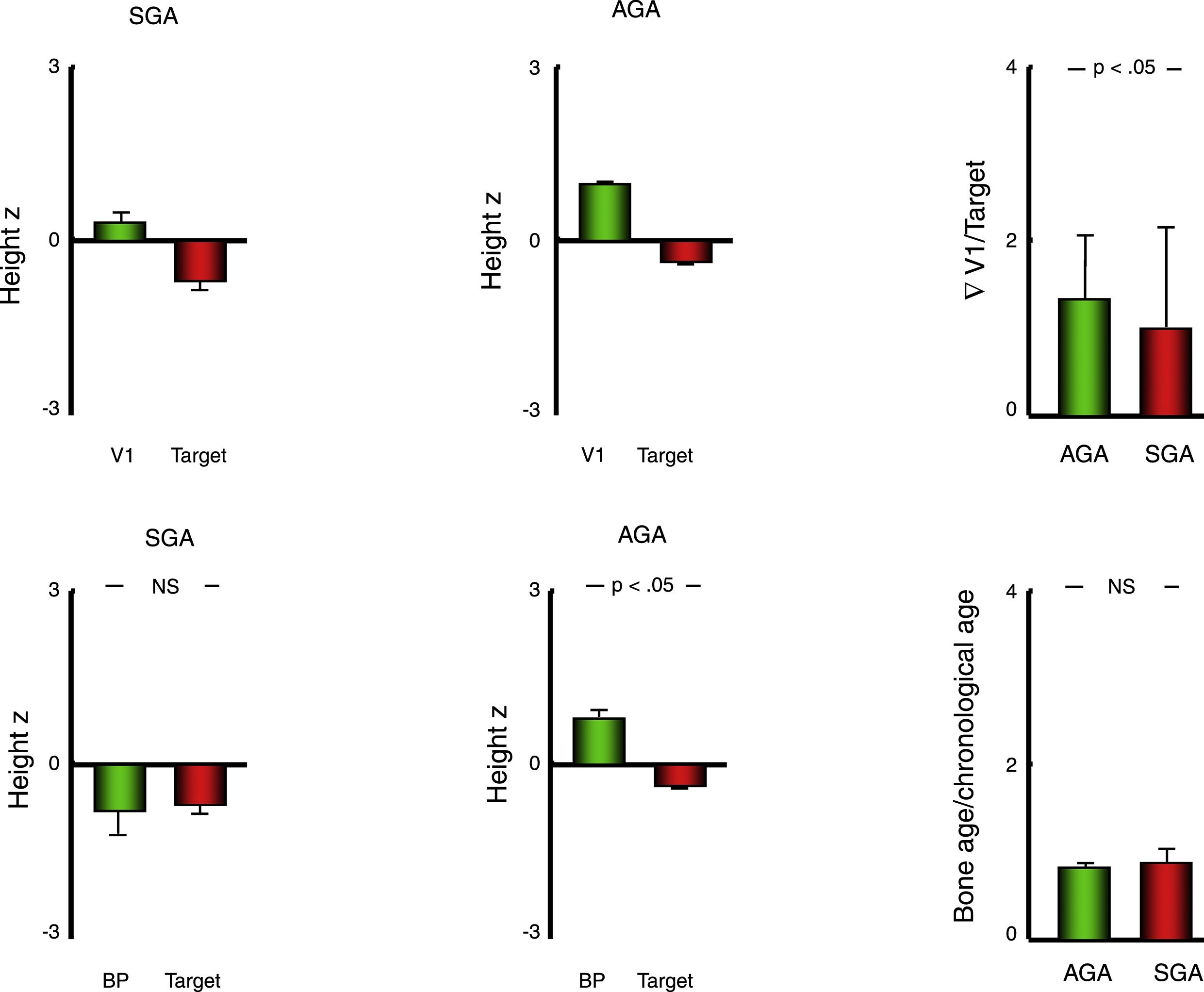
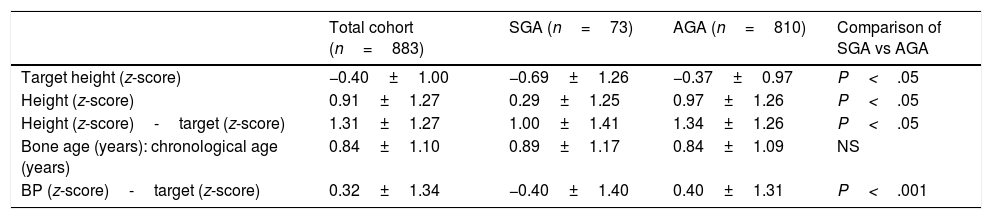
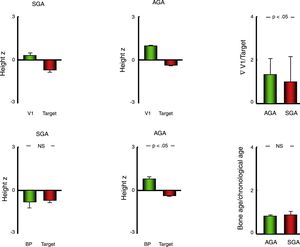
Linear growth and bone ageThe mean standardised height in the AGA group was higher compared to the SGA group (P<.001), but so was the standardised target height (P<.05) (Table 2). For this reason, we compared the linear growth parameters in each group relative to the target height of the patients.
Characteristics of growth in the cohort under study.
| Total cohort (n=883) | SGA (n=73) | AGA (n=810) | Comparison of SGA vs AGA | |
|---|---|---|---|---|
| Target height (z-score) | −0.40±1.00 | −0.69±1.26 | −0.37±0.97 | P<.05 |
| Height (z-score) | 0.91±1.27 | 0.29±1.25 | 0.97±1.26 | P<.05 |
| Height (z-score)-target (z-score) | 1.31±1.27 | 1.00±1.41 | 1.34±1.26 | P<.05 |
| Bone age (years): chronological age (years) | 0.84±1.10 | 0.89±1.17 | 0.84±1.09 | NS |
| BP (z-score)-target (z-score) | 0.32±1.34 | −0.40±1.40 | 0.40±1.31 | P<.001 |
Values standardised using the population-based growth charts of Hernández et al.18 as reference.
AGA, patients born with anthropometric values appropriate for gestational age; BP, predicted height estimated by the Bayley–Pinneau method; SGA, patients born with anthropometric values small for gestational age.
In so doing, we found that all patients had a height z-score significantly above their target height both overall (mean, +1.31; SD, 1.27), and in each group (SGA: +1.00±1.41; AGA: +1.34±1.26) (P<.001 in all comparisons), although this difference was significantly greater in the AGA group compared to the SGA group (P<.05) (Table 2) (Fig. 2).
Bar charts presenting the values of variables used to assess growth and bone age in patients with a history of born AGA or SGA. Data expressed as mean±standard deviation. AGA, patients born with anthropometric values appropriate for gestational age; BP, predicted final height estimated with the Bayley–Pinneau method; SGA, patients born with anthropometric values small for gestational age; V1, height in the first visit; z, z-score.
Furthermore, we found a significantly advanced bone age relative to chronological age both in the overall cohort (+0.84±1.10 years) and in the two groups (SGA, +0.89±1.17 years; AGA, +0.84±1.09) (P<.001 in all comparisons), although in this case we did not find a difference in the extent of the advancement between the SGA and AGA groups. For this reason, the predicted final height (BP) exceeded the target height in patients born AGA (P<.001) but not in patients born SGA (Table 2; Fig. 2).
At the end of the 5-year follow-up, we had been able to record the final height (growth velocity<1cm/year in the previous year) of 104 patients, corresponding to 5 in the SGA group and 99 in the AGA group. Consistently with the differences between groups in the predicted final height, the mean difference between the final height and the target height was of +1.01±5.31cm (z-score difference, +0.17±0.91) in the AGA group and −4.93±9.74cm (z-score difference, −0.81±1.62) in the SGA group.
DiscussionOur study, conducted in a large cohort of patients with obesity, found an association between a history of SGA and greater severity of metabolic comorbidities (insulin resistance and triglyceride levels) as well as poorer final height outcomes relative to the target height compared to patients with obesity of similar severity born AGA.
Low birth weight is one of the main causes of morbidity in the neonatal period and, along with postnatal catch-up growth, is associated with an increased risk of diseases later in life. Chief among these diseases are abnormalities in body composition, and it is known that greater weight gain in early childhood is associated with an increased risk of central adiposity.23,24 In children born SGA in particular, there is evidence of increased accumulation of visceral and hepatic fat at age 6 years compared to children of the same age and similar BMI born AGA,25 and it has been hypothesised that catch-up growth after intrauterine growth restriction results in increased adiposity during nutrition therapy that may in itself favour the development of insulin resistance due to an accumulation of fat mass as opposed to development of lean mass.4,26 It is likely that this increased adiposity, possibly predominantly visceral, plays a relevant role in the differences in metabolism observed between groups in our cohort in patients with similar BMI z-scores.
Our findings of greater severity of insulin resistance and higher levels of triglycerides in patients with a history of SGA are consistent with previous reports of an inverse correlation between birth weight and the prevalence of insulin resistance and MS in children and young adults,27 which was also suggested by the increased prevalence of MS observed in our study in the SGA group compared to the AGA group. Furthermore, the increased resistance to the activity of insulin in relation to extrauterine weight gain during catch-up growth is an early feature that can be identified in the first 2 years of life, and their association is further supported by the fact that this insulin resistance is observed nearly exclusively in children born SGA that experience spontaneous catch-up growth as opposed to children born AGA or children born SGA that do not exhibit catch-up growth.28
Another relevant finding was that 25-hydroxyvitamin D levels were lower in the patients born SGA, which is consistent with the hypothesis that being born SGA is a risk factor for vitamin D deficiency.29,30 Furthermore, since this vitamin is fat-soluble, the higher proportion of body fat in patients born SGA (relative to individuals born AGA of similar BMI z-scores) could be related to the differences found between the two groups under study.31 At any rate, given the many factors that influence circulating levels of vitamin D, especially in obese individuals, we ought to acknowledge the complexity involved in the analysis of these differences, especially taking into account relevant limitations, such as the variability in the seasons when blood samples were obtained from patients (we did not document the season of sample collection in our study, which is one of its limitations), which may be particularly significant in a cohort this large recruited over such a long period of time.
One novel aspect of our study is the assessment of the impact on growth of the combination of 2 medical conditions (anthropometric values consistent with SGA and childhood obesity) that have opposing effects on it. Thus, children born SGA tend to exhibit shorter heights during childhood and adolescence and achieve shorter final heights,32 while obese children usually experience more rapid prepubertal growth associated with an acceleration of bone maturation of varying degree that may result in a final height that nears or slightly exceeds the target height.17 The group of patients with obesity in our cohort seems to fit the latter growth pattern (all of them, whether born SGA or AGA, exhibited a height z-score in excess of the target height as well as advanced bone age), although the history of SGA involves a lesser degree of “overgrowth” relative to the target height compared to patients born AGA, which, combined with an equal degree of acceleration of bone maturation, makes the predicted height of individuals born SGA (relative to the target height) shorter compared to individuals born AGA, which was corroborated by the height outcomes of patients that achieved their final heights in our cohort.
It is likely that this finding is largely attributable to the process of recovery from the prenatal growth abnormalities, which may reduce the probability of children born SGA achieving a final height consistent with final heights in their family proportionally to how short their length is at birth. In addition, patients born SGA exhibit abnormalities in the adrenal and gonadal axis during prenatal development that may lead to abnormalities in adrenarche33 and pubertal development,34,35 resulting in more rapid pubertal growth which, combined with the rapid bone maturation, may in turn lead to final heights below the target height.36
In conclusion, our findings in a large cohort of obese paediatric patients suggest an association between the anthropometric factor of a history of SGA, a shorter predicted height and more severe disorders of lipid and carbohydrate metabolism in comparison with patients with obesity born with anthropometric measures AGA.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: González-Leal R, Martínez-Villanueva J, Argente J, Martos-Moreno GÁ. Influencia de la antropometría neonatal sobre las comorbilidades del paciente con obesidad. An Pediatr (Barc). 2019;90:362–369.
Previous presentation: This study was presented at the XXXVIICongress of the Sociedad Española de Endocrinología Pediátrica (SEEP), 2015, Valencia, Spain.