Congenital heart disease (CHD) represents the most common congenital malformation. The objective of this study was to analyse the incidence of CHD in Spain, and it is the first nationwide study so far.
MethodsA retrospective observational study was performed in order to evaluate the incidence of CHD in Spain. The administrative database (minimum basic data set) from 2003 to 2012 was analysed in children less than one year old admitted to hospital with codes of CHD (International Classification of Diseases, 9th Revision, clinical modification). Cumulative incidence, incidence relative risk, and standardised incidence ratio were calculated to study geographic variations.
ResultsThere were 64831 infants with CHD among the 4766325 births analysed during the period studied, with an incidence of 13.6‰. The incidence excluding atrial septal defect was 7.29 ‰. The most frequent CHD were atrial septal defect (6.31‰), ventricular septal defect (3.48‰), patent ductus arteriosus (2.71‰), coarctation of the aorta (0.55‰), pulmonary stenosis (0.50‰), transposition of the great arteries (0.49‰), atrioventricular septal defect (0.45‰), and tetralogy of Fallot (0.41‰).
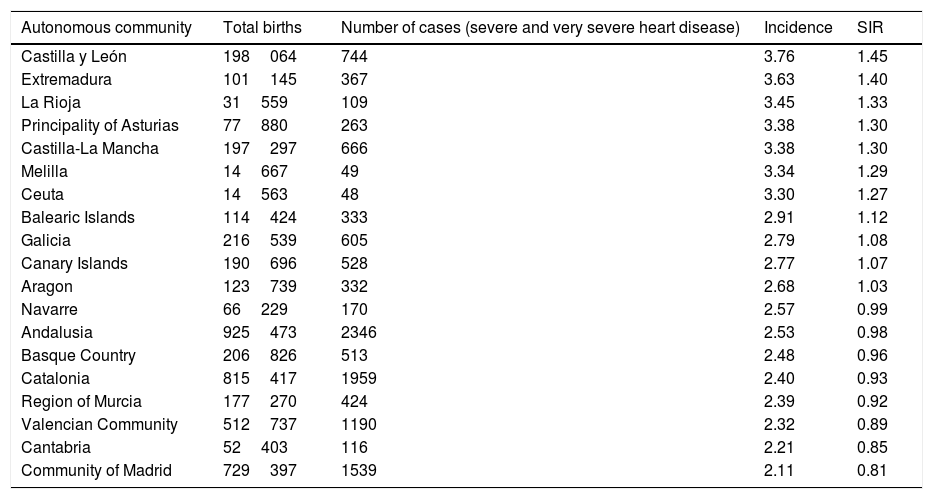
Castilla y León, together with Extremadura, showed the highest risks for severe and very severe CHD, while Madrid and Cantabria showed the lowest.
ConclusionsAn increase of mild CHD was observed during the analysed period. This could have been influenced by improvements in diagnostic techniques, extended use of echocardiography, and the International Classification of Diseases, 9th Revision, clinical modification coding system, and a decrease in very severe CHD, which is less influenced by external factors. Significant geographical differences were found in the incidence of severe and very severe CHD.
Las cardiopatías congénitas (CC) son las malformaciones congénitas más frecuentes. En España no hay datos de su incidencia a nivel nacional. El objetivo del estudio es analizar la incidencia y evolución de las CC en España.
MétodosEstudio observacional retrospectivo utilizando el conjunto mínimo básico de datos durante 10 años (2003-2012), en menores de un año, seleccionando las altas hospitalarias con códigos de CC (Clasificación Internacional de Enfermedades, 9.a revisión, modificación clínica). Se describió la evolución anual de la incidencia acumulada y su distribución geográfica, y se analizó mediante riesgos relativos de incidencia y razón de incidencias estandarizadas por comunidad autónoma.
ResultadosDurante el periodo analizado 64.831 menores de un año fueron diagnosticados de CC al alta hospitalaria sobre 4.766.325 nacimientos con una incidencia del 13,6‰. La incidencia excluyendo la comunicación interauricular fue del 7,29‰. Los códigos más frecuentes fueron: comunicación interauricular (6,31‰), comunicación interventricular (3,48‰), ductus arteriosus persistente (2,71‰), coartación de aorta (0,55‰), estenosis pulmonar (0,50‰), trasposición de grandes vasos (0,49‰), canal auriculoventricular (0,45‰) y tetralogía de Fallot (0,41‰).
La distribución geográfica de las cardiopatías graves y muy graves no resultó uniforme presentando Castilla y León junto con Extremadura la mayor incidencia, y Madrid y Cantabria la menor.
ConclusionesDurante el tiempo de estudio se observa un aumento de cardiopatías leves que puede estar influido por la mejora de las técnicas diagnósticas, el uso extendido de la ecocardiografía y la propia codificación Clasificación Internacional de Enfermedades, 9.a revisión, modificación clínica y una disminución de las cardiopatías muy graves cuyo análisis presenta mayor validez al estar menos influido por factores externos. La incidencia de cardiopatías graves y muy graves no fue uniforme en España.
Congenital heart defects (CHDs) are the most frequent type of congenital anomalies,1 with an approximate incidence of 8–10 cases per 1000 live births, which varies significantly between published studies.2–4 Factors contributing to this variability may be the age at diagnosis, differences in the definition of CHDs, the diagnostic methods used and the implementation of routine screening programmes. When it comes to Spain, there are studies published in different periods that used different methodologies, reporting an incidence of 8.96‰ in Navarre,5 of 5.4‰ to 16.1‰ in Badajoz,6 7.5‰ in Asturias7 and 13.4‰ in Valencia,8 and none reporting nationwide data.
The aim of our study was to analyse the incidence of the different types of CHDs in infants aged less than 1 year in Spain, their geographical distribution and their evolution over time between 2003 and 2012.
MethodsWe conducted a retrospective observational study, collecting data from the nationwide Minimal Basic Dataset (MBDS) population health care register for a period of 10 years (01/01/2003–31/12/2012). We selected hospital discharges in infants aged less than 1 year where the primary diagnosis and/or any of the secondary diagnoses corresponded to any of the codes for CHDs in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM), which are the following: 745 (bulbus cordis anomalies and anomalies of cardiac septal closure), 746 (other congenital anomalies of heart) or 747 (other congenital anomalies of circulatory system).
We excluded medical conditions that could be physiological and anomalies of the circulatory system that did not necessarily involve a CHD, such as patent ductus arteriosus (PDA) in preterm newborns, absent or hypoplastic umbilical artery, single umbilical artery, congenital heart block or unspecified anomalies.
We excluded instances of hospital readmission by comparing the sex, date of birth, health record number and hospital of all the selected discharge reports.
We calculated the number of new cases, taking into account only one episode per patient. Since the ICD-9 CM includes some nonspecific codes that cannot be analysed in isolation, for the purpose of determining the incidence of the main CHDs we aggregated these codes under the umbrella category of “other” (codes 745.7, 745.8, 745.9, 746.00, 746.09, 746.84, 746.87, 746.89, 746.9, 747.20, 747.21, 747.29, 747.3, 747.40).
We classified patients into 3 groups based on the severity of their disease, adapting our data to the classification used by the EUROCAT group2 (Table 1).
Classification of congenital heart defects by severity.
| Very severe heart disease |
| Single ventricle |
| Hypoplastic left heart |
| Atresia of pulmonary valve with intact septum |
| Ebstein anomaly |
| Tricuspid atresia |
| Severe heart disease |
| Pulmonary valve atresia |
| Common arterial truncus |
| Atrioventricular septal defect |
| Aortic valve stenosis |
| Transposition of great vessels |
| Tetralogy of Fallot |
| Total anomalous pulmonary venous return |
| Coarctation of aorta |
| Double outlet right ventricle |
| Cor triatriatum |
| Subaortic valve stenosis |
| Malformations of coronary arteries |
| Aortic valve atresia |
| Interrupted aortic arch |
| Partial anomalous pulmonary venous return |
| Mild heart disease |
| Ventricular septal defect |
| Atrial septal defect |
| Pulmonary valve stenosis |
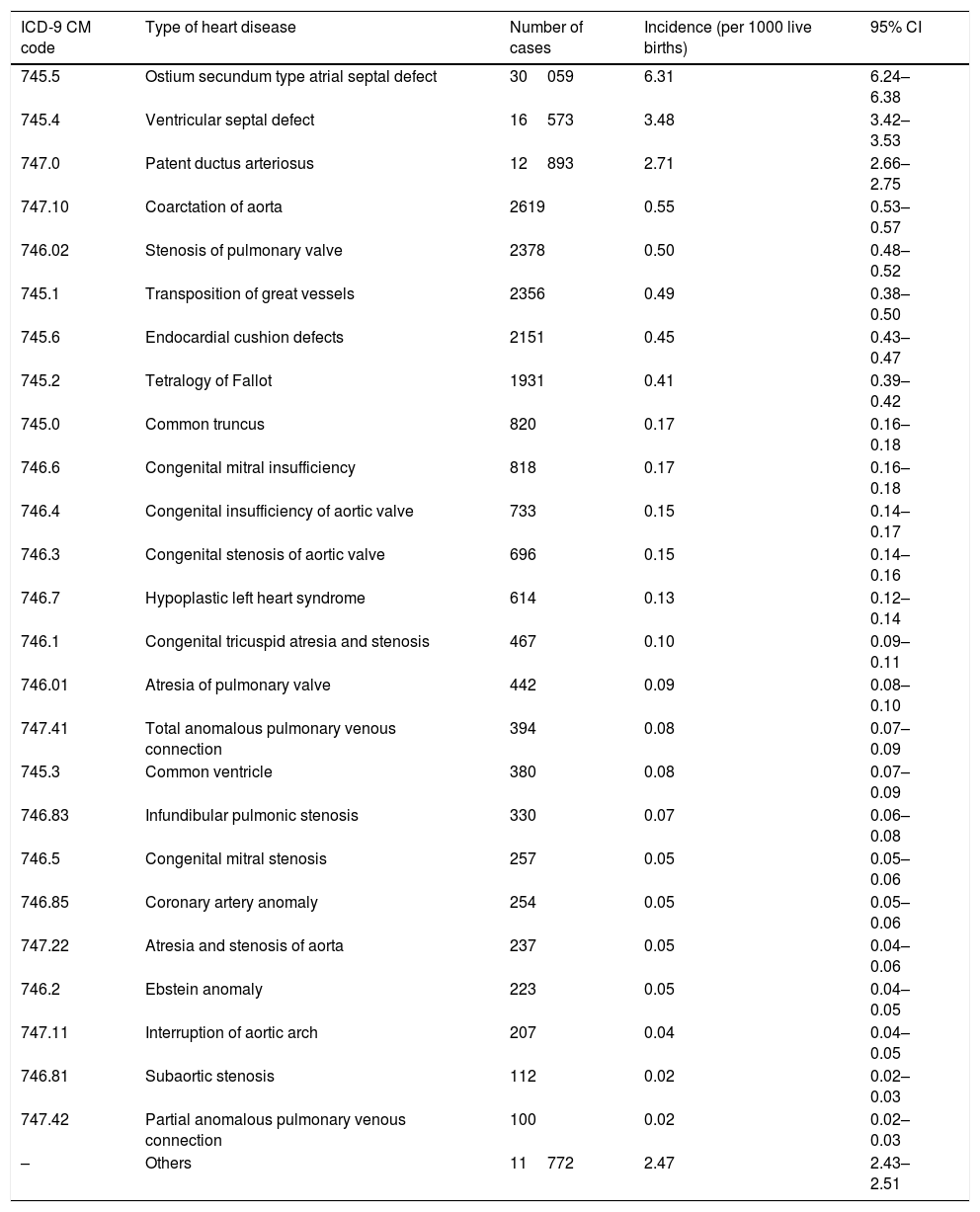
Table 2 lists the 26 ICD-9 CM codes used in the analysis.
Number of cases and incidence of each type of congenital heart defect by ICD-9 CM diagnostic code.
| ICD-9 CM code | Type of heart disease | Number of cases | Incidence (per 1000 live births) | 95% CI |
|---|---|---|---|---|
| 745.5 | Ostium secundum type atrial septal defect | 30059 | 6.31 | 6.24–6.38 |
| 745.4 | Ventricular septal defect | 16573 | 3.48 | 3.42–3.53 |
| 747.0 | Patent ductus arteriosus | 12893 | 2.71 | 2.66–2.75 |
| 747.10 | Coarctation of aorta | 2619 | 0.55 | 0.53–0.57 |
| 746.02 | Stenosis of pulmonary valve | 2378 | 0.50 | 0.48–0.52 |
| 745.1 | Transposition of great vessels | 2356 | 0.49 | 0.38–0.50 |
| 745.6 | Endocardial cushion defects | 2151 | 0.45 | 0.43–0.47 |
| 745.2 | Tetralogy of Fallot | 1931 | 0.41 | 0.39–0.42 |
| 745.0 | Common truncus | 820 | 0.17 | 0.16–0.18 |
| 746.6 | Congenital mitral insufficiency | 818 | 0.17 | 0.16–0.18 |
| 746.4 | Congenital insufficiency of aortic valve | 733 | 0.15 | 0.14–0.17 |
| 746.3 | Congenital stenosis of aortic valve | 696 | 0.15 | 0.14–0.16 |
| 746.7 | Hypoplastic left heart syndrome | 614 | 0.13 | 0.12–0.14 |
| 746.1 | Congenital tricuspid atresia and stenosis | 467 | 0.10 | 0.09–0.11 |
| 746.01 | Atresia of pulmonary valve | 442 | 0.09 | 0.08–0.10 |
| 747.41 | Total anomalous pulmonary venous connection | 394 | 0.08 | 0.07–0.09 |
| 745.3 | Common ventricle | 380 | 0.08 | 0.07–0.09 |
| 746.83 | Infundibular pulmonic stenosis | 330 | 0.07 | 0.06–0.08 |
| 746.5 | Congenital mitral stenosis | 257 | 0.05 | 0.05–0.06 |
| 746.85 | Coronary artery anomaly | 254 | 0.05 | 0.05–0.06 |
| 747.22 | Atresia and stenosis of aorta | 237 | 0.05 | 0.04–0.06 |
| 746.2 | Ebstein anomaly | 223 | 0.05 | 0.04–0.05 |
| 747.11 | Interruption of aortic arch | 207 | 0.04 | 0.04–0.05 |
| 746.81 | Subaortic stenosis | 112 | 0.02 | 0.02–0.03 |
| 747.42 | Partial anomalous pulmonary venous connection | 100 | 0.02 | 0.02–0.03 |
| – | Others | 11772 | 2.47 | 2.43–2.51 |
CI, confidence interval; ICD-9 CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
The reference population we used for calculations of disease incidence was the census of births of the Instituto Nacional de Estadística (National Institute of Statistics, http://www.ine.es/) for each year under study.
In the descriptive study of the patients, we summarised qualitative variables as frequency distributions and compared them by means of the chi square test or the Fisher exact test. We described quantitative variables using the mean and standard deviation and calculated the corresponding 95% confidence intervals (CIs).
We calculated the cumulative incidence as the proportion of new cases of CHD in the first year of life over the total at-risk population at the beginning of the follow-up period.
We calculated incidences for the total population, by autonomous community, by sex and by severity group for the 2003–2012 period. We expressed cumulative incidences in cases per 1000 births (‰), calculating their corresponding confidence intervals.
We compared the annual incidence of CHDs relative to all live births by means of Poisson regression using the 2003 cohort as the reference group. We calculated the annual risk ratios with the corresponding 95% CIs.
We calculated standardised incidence ratios by comparing the number of cases observed in each region with the number expected under the assumption that the incidence was similar to the Spanish mean, as well as their 95% CIs. We used the direct method to standardise rates.
We processed the collected data using MS Access and then exported them to the software SPSS version 17 for subsequent analysis. We calculated the CIs of rates using the software Open Source Epidemiologic Statistics for Public Health (OpenEpi).
ResultsIn the period under study, a total of 64831 infants out of a cohort of 4766325 live births in Spain received a diagnosis of CHD.
The sex distribution was 53.43% boys and 46.57% girls, and the difference in proportions was statistically significant (P<.001).
The mean age at diagnosis was 36.2 days (standard deviation, 75.3 days), and 68.3% of patients received a diagnosis within 10 days from birth.
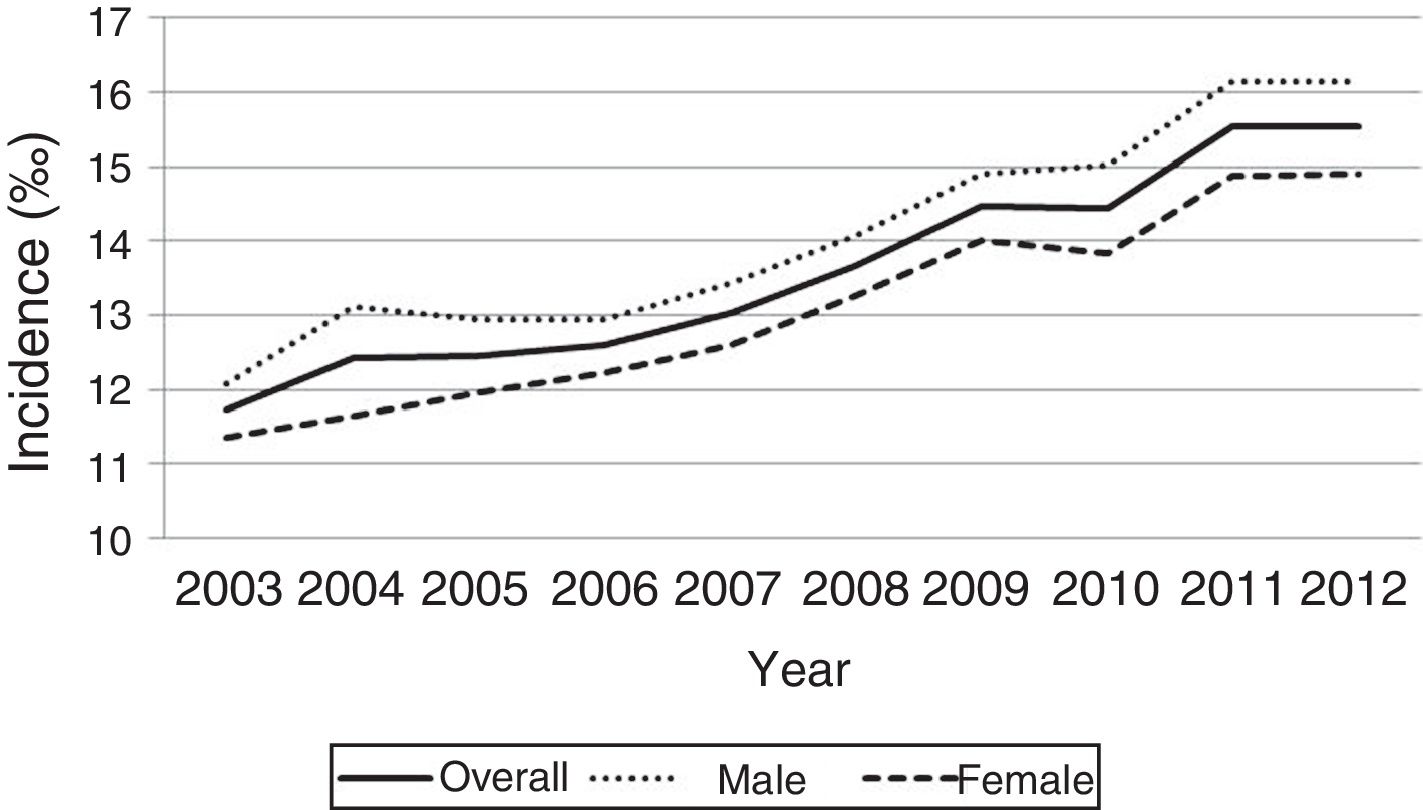
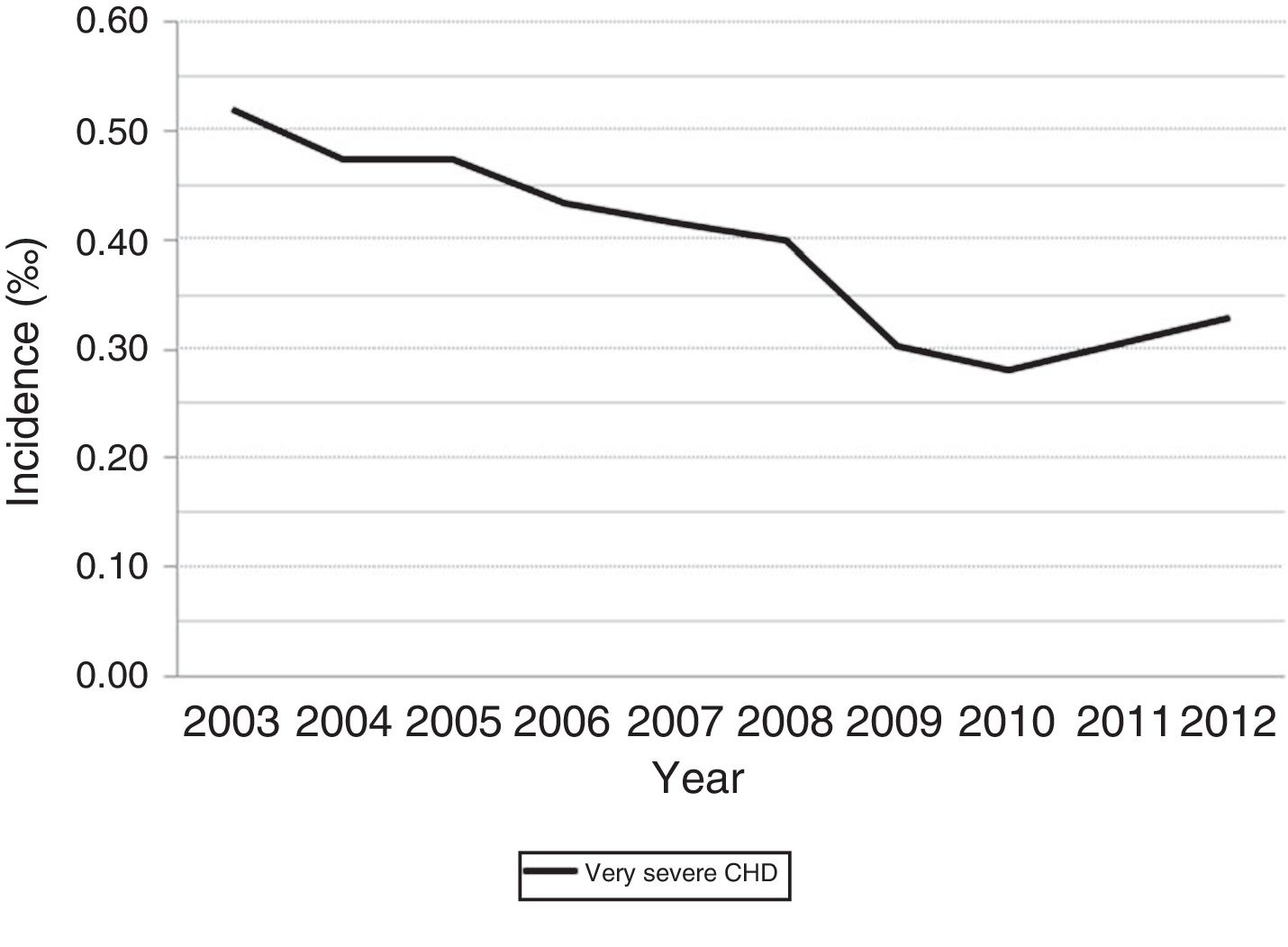
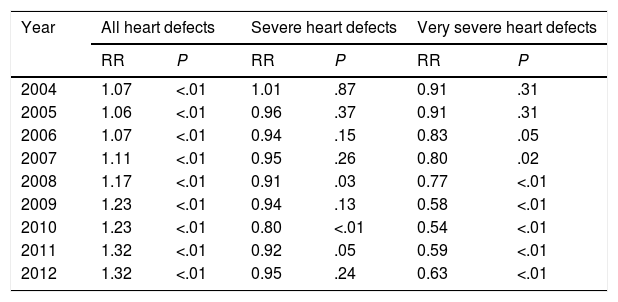
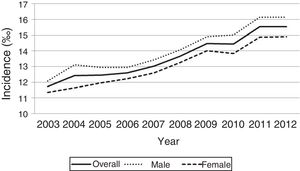
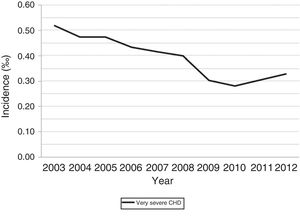
The incidence we found using the ICD-9 CM codes was 13.6‰ (95% CI, 13.5–13.7). When we excluded atrial septal defects (ASDs), which was the most frequent CHD in our series, the incidence was reduced to 7.29‰. Fig. 1 shows the evolution of the annual incidence, overall and by sex. Table 2 shows the distribution by type of CHD along with the cumulative incidence. Fig. 2 shows the annual evolution of the incidence of very severe CHDs in the years under study. Table 3 shows the risk ratios (of incidence proportions) of CHD, overall and for severe and very severe defects, for each year under study. Table 4 shows the standardised incidence ratio of severe and very severe CHDs by autonomous community, calculated based on the national mean, that is, the expected value if the risk corresponded to the mean risk for all of Spain.
Risk ratios of congenital heart defects (overall, severe and very severe) by year under study.
| Year | All heart defects | Severe heart defects | Very severe heart defects | |||
|---|---|---|---|---|---|---|
| RR | P | RR | P | RR | P | |
| 2004 | 1.07 | <.01 | 1.01 | .87 | 0.91 | .31 |
| 2005 | 1.06 | <.01 | 0.96 | .37 | 0.91 | .31 |
| 2006 | 1.07 | <.01 | 0.94 | .15 | 0.83 | .05 |
| 2007 | 1.11 | <.01 | 0.95 | .26 | 0.80 | .02 |
| 2008 | 1.17 | <.01 | 0.91 | .03 | 0.77 | <.01 |
| 2009 | 1.23 | <.01 | 0.94 | .13 | 0.58 | <.01 |
| 2010 | 1.23 | <.01 | 0.80 | <.01 | 0.54 | <.01 |
| 2011 | 1.32 | <.01 | 0.92 | .05 | 0.59 | <.01 |
| 2012 | 1.32 | <.01 | 0.95 | .24 | 0.63 | <.01 |
RR, risk ratio (incidence proportions).
Standardised risk ratio by autonomous community based on the nationwide mean incidence of severe and very severe congenital heart defects.
| Autonomous community | Total births | Number of cases (severe and very severe heart disease) | Incidence | SIR |
|---|---|---|---|---|
| Castilla y León | 198064 | 744 | 3.76 | 1.45 |
| Extremadura | 101145 | 367 | 3.63 | 1.40 |
| La Rioja | 31559 | 109 | 3.45 | 1.33 |
| Principality of Asturias | 77880 | 263 | 3.38 | 1.30 |
| Castilla-La Mancha | 197297 | 666 | 3.38 | 1.30 |
| Melilla | 14667 | 49 | 3.34 | 1.29 |
| Ceuta | 14563 | 48 | 3.30 | 1.27 |
| Balearic Islands | 114424 | 333 | 2.91 | 1.12 |
| Galicia | 216539 | 605 | 2.79 | 1.08 |
| Canary Islands | 190696 | 528 | 2.77 | 1.07 |
| Aragon | 123739 | 332 | 2.68 | 1.03 |
| Navarre | 66229 | 170 | 2.57 | 0.99 |
| Andalusia | 925473 | 2346 | 2.53 | 0.98 |
| Basque Country | 206826 | 513 | 2.48 | 0.96 |
| Catalonia | 815417 | 1959 | 2.40 | 0.93 |
| Region of Murcia | 177270 | 424 | 2.39 | 0.92 |
| Valencian Community | 512737 | 1190 | 2.32 | 0.89 |
| Cantabria | 52403 | 116 | 2.21 | 0.85 |
| Community of Madrid | 729397 | 1539 | 2.11 | 0.81 |
SIR, standardised incidence ratio.
The table shows the expected values should the risk equal the median for all of Spain; values above 1 indicate a greater than expected risk.
We found a male-to-female ratio of 1.15. The higher incidence in boys is not a consistent finding in the literature, as it has been reported by some authors,9 while others have described a slightly higher incidence in girls.3 In our study, the probability of CHD occurring in a male patient was significantly greater.
The incidence we found in our study (13.6‰) was noticeably higher than the traditional estimate10 of 8‰. However, in recent years studies have been published2,9 that reported incidences of 11‰ and even higher2 in countries such as Switzerland (13.43‰) or Malta (14.7‰).
In Spain, studies have been published that reported an incidence of 8.96‰ in Navarre for the 1989–1998 period,5 an incidence of 7.5‰ in Asturias for the 1990–2004 period7 and an incidence of 13.4‰ for Valencia in 2012.8
We must compare these results with caution, given the differences in the methodology employed, especially in the studies conducted in Navarre and Asturias. Our study can be compared more safely with the one conducted in Valencia, given the similar time frame and methodology, and both had similar results.
In the period under study, there was an increase in the incidence of mild CHDs, from 6.49‰ in 2003 to 9.93‰ in 2012, with no significant changes in the incidence of severe CHDs (2.34‰ in 2003 and 2.22‰ in 2012) and a clear decline in very severe CHDs (0.52‰ in 2003 and 0.33‰ in 2012) (Fig. 2), findings that were consistent with the literature.11
This increase in cases of mild CHDs probably reflects improvements in diagnosis rather than an actual increase in incidence. The widespread use of echocardiography in neonatal units to assess newborns with respiratory problems or nonspecific heart murmurs can lead to the identification of trivial and asymptomatic lesions that frequently resolve spontaneously in the early months of life, resulting in a disproportionate increase in the overall incidence of CHDs on account of these mild forms.
In nearly 70% of the patients in our study, the defects were diagnosed within 10 days from birth, a timeframe when it is possible to detect ASDs, ventricular septal defects (VSDs) or small PDAs that close spontaneously in the early months of life but documented at discharge as cases of CHD, an issue that must be taken into account in data analysis. Using routine echocardiographic screening in a sample of 5190 newborns, Zhao et al.12 found an incidence of CHD of 26.6‰ that declined to 19.5‰ at 4 months of followup, due in large part to the spontaneous closure of small muscular VSDs. A high rate of spontaneous closure of ASDs has also been reported.13
The most frequent type of CHD in our study was ASD (33.55%), with an incidence of 6.31‰. Although the reported incidence varies widely between studies in the literature,14 estimates usually range between 0.3‰ and 4.2‰. Some studies12 found an incidence similar to the one in our study, although they used routine echocardiographic screening in all newborns. The study by Cavero et al.,8 conducted in the Valencian Community, found an incidence of 5.9‰, slightly lower than the one found in our study.
The diagnosis of ostium secundum type ASD using the ICD-9 CM scheme presents a significant drawback, as the 745.5 code, “ostium secundum type ASD,” includes patent foramen ovale and does not differentiate between the two diagnoses. Patent foramen ovale is a very frequent finding in the first weeks of life, and its inclusion in this code results in increases in the overall documented incidence of CHDs and ASD. Methods have been proposed to attempt to correct the vagueness of codes in the ICD-9 CM in the adult population by associating the diagnostic codes with the corresponding codes for surgical procedures or interventions to treat haemodynamic instability. These methods cannot be applied to our study, which was restricted to infants aged less than 12 months that may have been eligible for surgical repair after 1 year of age. Consequently, the ICD-9 CM is of limited value for the estimation of the incidence of CHDs overall and specifically of ASD, but this drawback does not affect other heart defects.
The second most frequent type of heart defect was VSD (18.9%). In our study, its incidence was 3.48‰, while the incidence reported in past large-scale studies ranges between 0.3‰ and 7.7‰.
The ICD-9 CM classification does not allow the subclassification of VSD based on size and uses the same code for large and very small defects, when a large proportion of the latter end up closing spontaneously.
The third most frequent heart defect in our case series was PDA (14.39%), with an incidence of 2.71‰, similar to that reported in previous large-scale studies.9
The diagnosis of PDA poses some challenges as to when it should be included in the count of CHDs. According to the International Pediatric and Congenital Cardiac Code,15 PDA should be considered a CHD when it persists past age 3 months. In our study, we may have overestimated the incidence of PDA, as nearly 70% of the patients received their diagnosis in the first 10 days of life.
On the other hand, cases of silent PDA should probably not be included in the calculation of the incidence of CHDs, but neither the ICD-9 CM nor the ICD-10 classification systems allow the distinction between silent and haemodynamically significant PDA.
The fourth most frequent CHD was coarctation of aorta (2.92%) with an incidence of 0.55‰, consistent with the literature,13 in which reported incidences range between 0.28‰ and 0.64‰.
The fifth leading CHD was pulmonary valve stenosis (2.65%), with an incidence of 0.50‰, close to the value reported in a German study,3 which estimated it at 0.66‰, and higher than the one reported by the EUROCAT group,16 which estimated it at 0.24‰ in the 2008–2012 period (http://www.eurocat-network.eu/).
Transposition of great vessels, with an incidence of 0.49‰, was the sixth most frequent heart defect, and within this diagnosis, the most frequent subcategory was complete transposition of great vessels (68.5%), followed by double outlet right ventricle (DORV) (23.9%), and corrected transposition of great vessels (4.6%).
In our study, DORV, with 563 cases, amounted to 0.63% of the total CHDs, with an incidence of 0.12‰. This was similar to the findings of the EUROCAT study for the 2008–2012 period,16 where the incidence was 0.92‰, corresponding to 271 cases.
The coding of cases of DORV using the ICD-9 CM may be inaccurate in relation to the clinical reality. This disease may present with different phenotypes, but administrative nomenclature forces the classification of all patients with DORV under a single code, precluding differentiation of clinical types.
In our study, tetralogy of Fallot was, in agreement with the historical literature, the most frequent cyanotic heart defect, with an incidence of 0.41‰, higher in comparison to complete transposition of great vessels (0.34‰). The study by de Egbe et al.,9 whose methodology was similar to ours in some aspects, found an incidence of tetralogy of Fallot of 0.28‰.
The incidence of other CHDs such as atrioventricular septal defect (0.45‰), common truncus (0.17‰), pulmonary valve atresia (0.09‰) and total anomalous pulmonary venous connection (0.08‰) was consistent with the literature.8,13 Within the category of endocardial cushion defects, the most frequent form in our case series was atrioventricular septal defect (73.8%) followed by ostium primum defect (24.6%).
Hypoplastic left heart syndrome, with an incidence of 0.13‰, was less frequent in comparison with the 0.2‰ incidence reported in the previous literature.13
The classification of CHDs by severity is useful in incidence studies, as mild lesions are the most frequent type by far and can contribute to the variability in the incidence reported in published studies.
As occurred in our study, several authors have found that the calculated incidence of CHDs depends mainly on the number of cases of mild defects17 and that the increase over time in the overall incidence of CHDs is mainly due to increases in the frequency of mild defects.18
In our study, the proportion of very severe CHDs was 3.6%, the proportion of severe CHDs was 20.4% and the proportion of mild CHDs was 75.8%. These findings are similar to those published by Dolk et al.2 in 2011, who found proportions of 4.4%, 21.5% and 63%, respectively.
When it came to the incidence by severity category in our series, the mean incidence in the 10 years under study was 0.39‰ for very severe defects, 2.19‰ for severe defects and 8.10‰ for mild defects.
We found a significant decline in the incidence of very severe forms of CHDs in the period under study (from 0.52‰ in 2003 to 0.33‰ in 2012), with a 37% decline in the risk ratio of being born with a very severe CHD in year 2012 relative to year 2003 (Table 3). We did not find significant differences over time in the group of severe CHDs.
The most severe forms of heart disease are diagnosed prenatally more often than milder forms19; for instance, the diagnosis of single ventricle or hypoplastic left heart syndrome is made prenatally in nearly 90% of cases.20
Most foetal echocardiography clinical practice guidelines recommend echocardiographic evaluation of the foetal thorax in the second trimester of pregnancy, including a 4-chamber view and outflow tract views. The more anomalous the anatomy visualised in the 4-chamber view, the more likely it is that an existing defect will be detected during a routine echocardiogram.
Similarly, lesions such as total anomalous pulmonary venous return, which in some cases may not significantly change the appearance of the anatomy in the 4-chamber view, are more likely to go undetected if this is the only view assessed in the echocardiogram.
In Spain, with the use of the ICD-9 CM system, it is not possible to determine the number of voluntary terminations of pregnancy (VTP) performed due to a foetal cardiac anomaly, as there is no specific code for this event.
In a study conducted in France in 2012, Khoshnood et al.4 found a relative frequency of VTP of 16.2% in a series of cases of CHDs diagnosed prenatally, so it would be fair to assume that a significant number of VTPs are performed in Spain due to the presence of a cardiac anomaly in the foetus, which would influence the proportion of very severe CHDs.
In our study, in 2012 and compared to 2003, nearly 70% fewer children were born with hypoplastic left heart syndrome, 70% fewer with tricuspid valve atresia and stenosis and 60% fewer with single ventricle. In the first period, there was a 35% decline in births of children with transposition of great vessels and a 14% increase in cases of total anomalous pulmonary venous return.
When we analysed the geographical distribution of severe and very severe heart defects in Spain, we found that it was not homogeneous, with the autonomous communities of Castilla y León and Extremadura having the highest incidence of severe and very severe defects, and the Community of Madrid and Cantabria the lowest.
When we standardised the incidence of each autonomous community based on the national mean, we found that the risk of being born with a severe or very severe CHD in Castilla y León was 1.45 greater compared to the rest of Spain, while in the following autonomous communities the risk was below the expected value: Navarre, Andalusia, Basque Country, Catalonia, Region of Murcia, Valencian Community, Cantabria and the Community of Madrid; in the latter, the risk of being born with a CHD was nearly 20% lower. We did not find any studies in the literature that had analysed these data at the national level with which we could compare our results.
When it comes to the limitations of our study, we ought to highlight that the information contained in the MBDS is drawn from clinical reports, which means that there is variability based on the level of detail of the authoring physician, the skills of the subsequent coder, and the correct entry of administrative variables.
The level of precision of the ICD-9 CM in describing CHDs may pose challenges, and the document authored by the Technical Unit on the ICD-9 CM and published by the Ministry of Health in 2008 can guide the coding of congenital heart defects.21
There are further limitations associated with the characteristics of the study and the database used. Thus, any cases diagnosed in clinics that did not result in hospital admission have been lost, as were any cases diagnosed after 1 year of age, although all of these would correspond to mild heart defects that do not require admission for diagnosis or treatment and that are not detected at birth.
In addition, some private hospitals do not submit the MBDS to the Ministry of Health.
We did not include cases of intrauterine death due to severe heart defects or VTPs due to cardiac malformations.
Some ICD-9 CM codes were not part of any of the 3 CHD severity categories considered in the study, and therefore were not included in the analysis.
In conclusion, the increase in the diagnosis of mild heart defects has probably been influenced by improvements in diagnostic methods, the widespread use of echocardiography and the very use of the ICD-9 CM coding system, factors that have a lesser effect on the estimation of the incidence of severe and very severe heart defects, whose analysis has greater validity as it is less influenced by external factors. The risk of being born with a CHD is not homogeneous throughout Spain.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez-Lescure Picarzo J, Mosquera González M, Latasa Zamalloa P, Crespo Marcos D. Incidencia y evolución de las cardiopatías congénitas en España durante 10 años (2003-2012). An Pediatr (Barc). 2018;89:294–301.