The aim of this study is to analyse changes in the incidents reported after the implementation of a new model, and study its results on patient safety.
Patients and methodsIn 2012 an observational study with prospective collection of incidents reported between 2007 and 2011 was conducted. In May 2012 a model change was made in order to increase the number of reports, analyse their causes, and improve the feedback to the service. Professional safety representatives were assigned to every department, information and diffusion sessions were held, and a new incident reporting system was implemented. With the new model, a new observational study with prospective collection of the reports during one year was initiated, and the results compared between models.
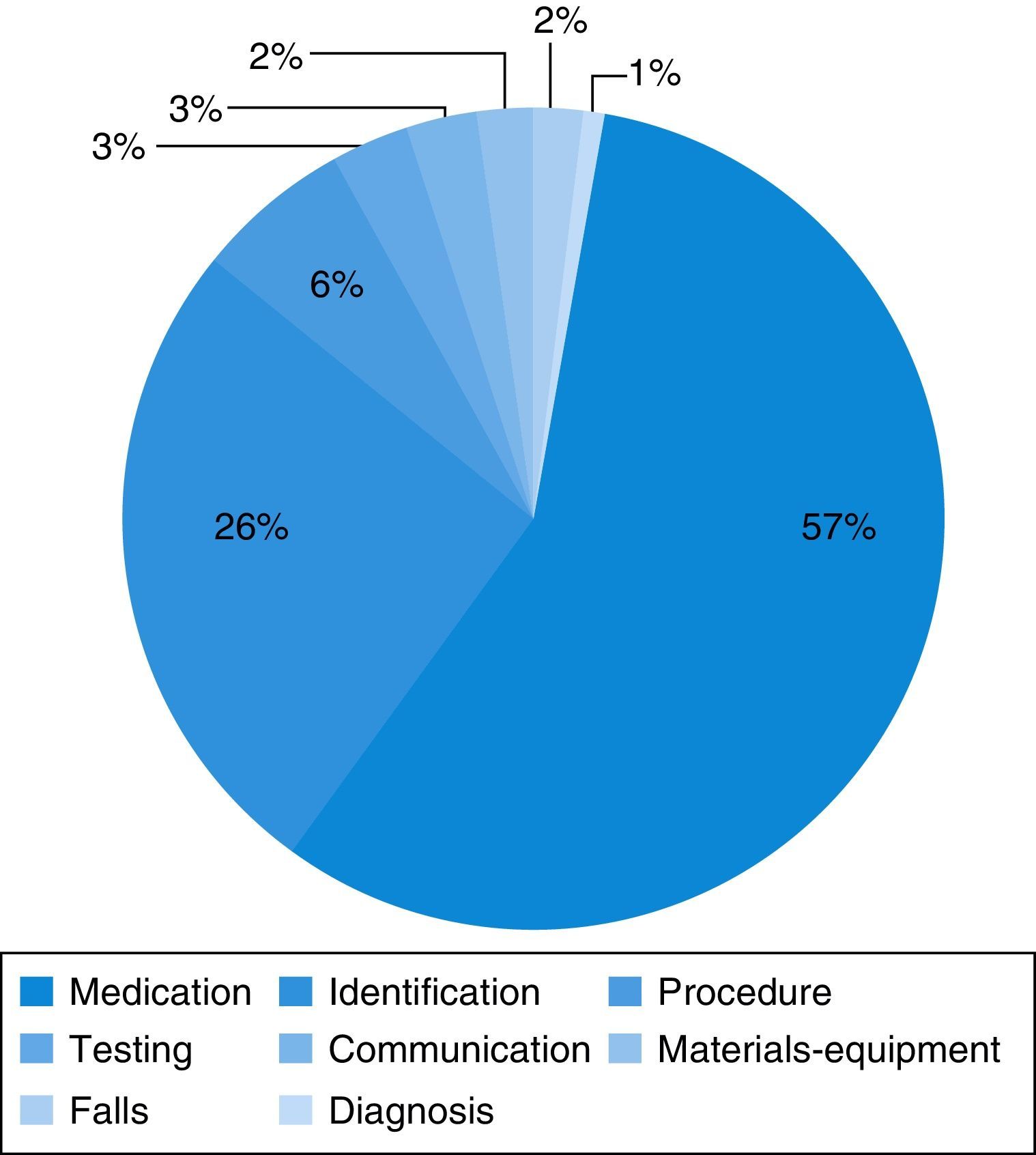
ResultsIn 2011, only 19 incidents were reported in the Emergency Department, and between June 1, 2012 to June 1, 2013, 106 incidents (5.6 times more). The incidents reported were medication incidents (57%), identification (26%), and procedures (7%). The most frequent causes were human (70.7%), lack of training (22.6%), and working conditions (15.1%). Some measures were implemented as a result of these incidents: a surgical checklist, unit doses of salbutamol, tables of weight-standardised doses of drugs for cardiopulmonary resuscitation.
ConclusionsThe new model of reporting incidents has enhanced the reports and has allowed improvements and the implementation of preventive measures, increasing the patient safety in the Emergency Department.
El objetivo del trabajo es analizar los cambios en la declaración de incidentes tras haber implantado un nuevo sistema de declaración y exponer las medidas aplicadas gracias a las declaraciones realizadas.
Pacientes y métodosEn el 2012 se realizó una recogida de los incidentes declarados de forma prospectiva entre 2007 y 2011. En mayo del 2012 se realizó un cambio de modelo para aumentar las declaraciones, analizar sus causas y mejorar el retorno de información al resto del equipo. Se nombraron referentes de seguridad en cada servicio, se realizaron sesiones informativas y de difusión, y se implantó un nuevo sistema de declaración de incidentes. Con el nuevo modelo se inició un estudio prospectivo de las declaraciones durante un año y se compararon los resultados con ambos modelos.
ResultadosEn todo el 2011 se declararon 19 incidentes en Urgencias y del 1 de junio de 2012 al 31 de mayo del 2013, 106 incidentes (5,6 veces más). Los incidentes declarados fueron de medicación (57%), identificación (26%) y procedimientos (7%). Las causas más frecuentes de estos fueron individuales del profesional (70,7%), falta de formación (22,6%) y condiciones de trabajo (15,1%). Medidas que se han aplicado a raíz de estos incidentes son el checklist quirúrgico, las monodosis de salbutamol y tablas por peso de fármacos de reanimación cardiopulmonar.
ConclusionesEl nuevo modelo de declaración de incidentes ha potenciado las declaraciones, ha permitido implantar mejoras y medidas preventivas, aumentando todo esto el clima de seguridad en el servicio de Urgencias.
Patient safety is an increasingly prioritised objective in the healthcare field. Several factors make emergency care settings particularly high-risk, including the stress caused by emergency situations, the high patient volume, the fast decision-making, the cumulative fatigue caused by long shifts, and the varying levels of experience of physicians.1
The particular characteristics of children make them especially vulnerable: dosages need to be calculated individually, often requiring special dilutions, children have a lower functional reserve to compensate errors, and are unable to convey their symptoms or discuss procedures and treatments.2
The first step in improving patient safety is creating a safety culture in the healthcare setting.3 Traditionally, it was thought that healthcare providers ought to be infallible and that errors involved negligence, so physicians would blame themselves for mistakes. Thus, it is essential that the culture of blaming individuals shifts to a new perspective in which mistakes are seen as opportunities for improving the system and preventing harm.4 A safety culture is based on the belief that errors do happen, that systems can be designed to reduce their frequency,5 and that they result from multiple factors or chains of events and can rarely be attributed to a single individual.6
One tool that has proven useful in the promotion of a safety culture is voluntary safety incident reporting combined with safety incident analysis and feedback, allowing the implementation of measures for improving the system.7,8
Voluntary error reporting systems have some advantages over the retrospective chart reviews and computerised error detection systems, such as the identification of administrative errors or the detection of “near misses”. “Near misses” are errors that do not reach the patient, yet constitute the base of the iceberg of all the incidents that occur, and they are very helpful for analysing the causes of errors and detecting flaws in the system.9
The analysis of safety incidents and subsequent feedback to the entire staff is very important to promote reporting. The process as a whole fosters a safety climate in healthcare settings.10
There are few studies analysing the incidence of errors in the paediatric population.
The primary objective of our study was to analyse the incidents reported in the Emergency Department over a one-year period and to present several measures developed on the basis of safety incident analysis that have been implemented in our department over time. Our secondary objective was to compare the incidents that occurred before and after the introduction of a new model for reporting incidents.
Patients and methodsWe conducted a study over a period spanning from before to after the implementation of a new model for safety incident reporting. The study took place in the emergency department of a tertiary children's hospital that receives about 100000 emergency room visits a year from children 0 to 18 years of age.
In 2012 we recorded the incidents that had been reported between 2007 and 2011, which was Period 1.
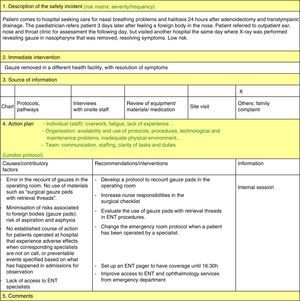
The pre-intervention model for reporting incidents comprised five different forms available online or in print that applied to the different types of events reported: allergic reactions, patient identification incidents, diet and medication incidents, and falls. Each form had different items to fill out (date, place, what happened, why, other specific items depending on the type of incident, etc.).
In May 2012 the model was changed to increase reporting and improve the feedback to the team. Professional safety representatives were appointed in each department, information sessions were held, and a new system for the reporting and management of safety incidents was introduced that consisted of:
- 1.
Voluntary reporting of incidents by means of an online form accessible to the whole staff through the hospital intranet and that allows for a broader range of incident types. Reporting through this system is voluntary, anonymous and nonpunitive.
- 2.
Notifying the Safety Unit representative.
- 3.
Management of the incident by the Safety Unit and risk matrix.
- 4.
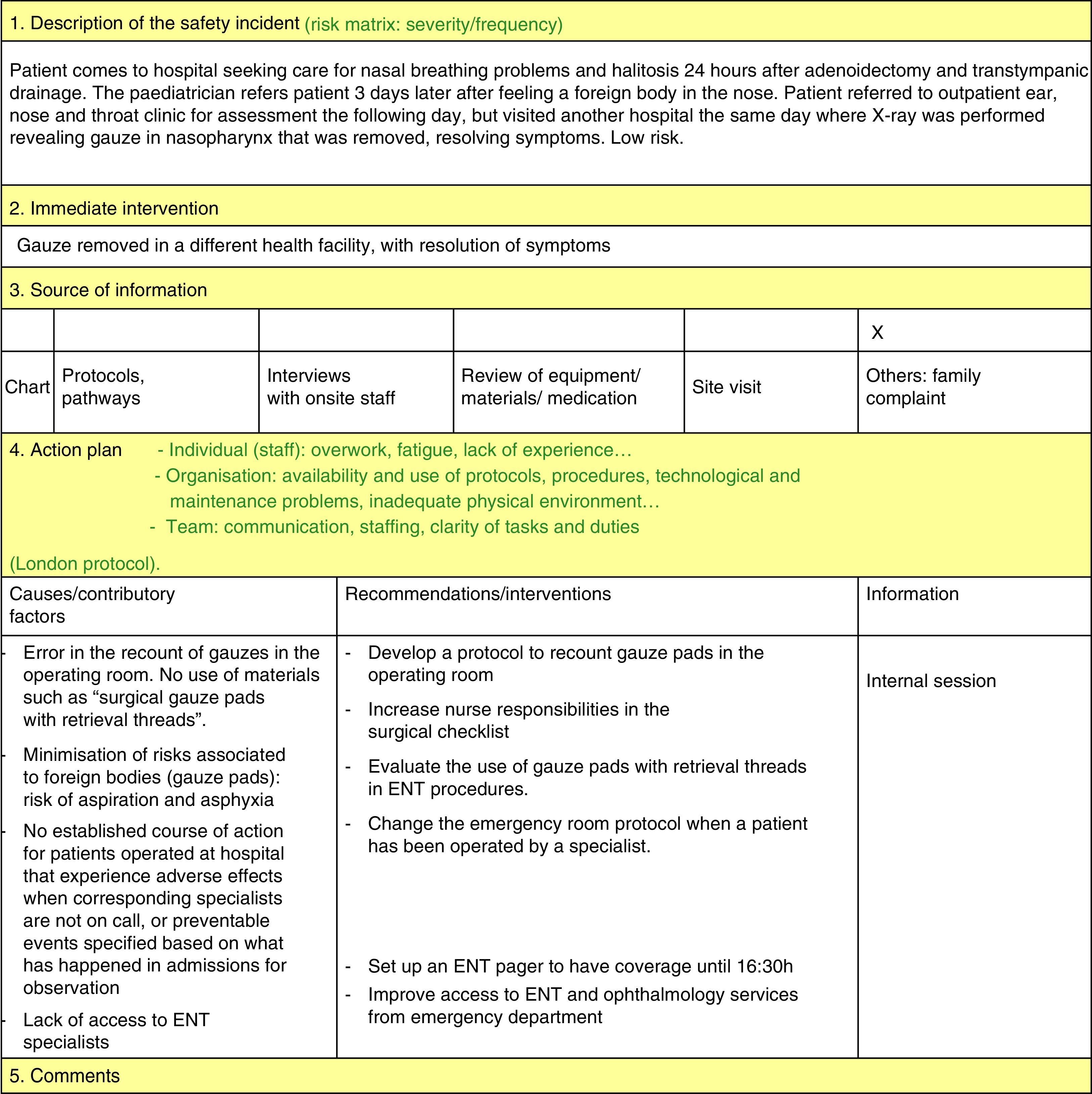
Safety incident analysis by the safety representatives of the department (ongoing as reports get submitted) by means of the system analysis of clinical incidents (London Protocol)11:
- 5.
Implementation of measures developed on the basis of the reported incidents.
- 6.
Assessment and ongoing monitoring of implemented measures.
- 7.
Feedback based on the data, delivered in monthly meetings and by the online diffusion of the minutes of these meetings to every health worker in the department to make them aware of the measures being taken and to encourage continued reporting.
Priorities were set for the implementation of improvement measures: measures that address incidents posing a higher risk to patients (these require an additional meeting with the involved departments to implement measures to prevent their recurrence), measures that are easiest to implement (for example, unit doses of salbutamol) and measures addressing the most frequent incidents (for example, nebulised salbutamol). These measures are reassessed and the staff informed on them periodically.
We undertook a prospective study by collecting the reports submitted under the new model between June 1, 2012 and June 1, 2013 (Period 2).
We defined the severity of the incidents based on the NPSA criteria13 as adapted by Lesar.14
The statistical analysis was performed with the SPSS software version 19.0. We did a descriptive study of quantitative variables expressed as median and interquartile range or mean±standard deviation, and of qualitative variables expressed as frequencies and percentages.
ResultsBetween June 2012 and June 2013 (Period 2), 106 incidents were reported in the paediatric emergency department, compared to 19 incidents reported in 2011 (reports increased by a factor of 5.7). During 2013, 102 incidents were reported in this department out of the total 630 reported for the entire hospital (16.1%).
Under the pre-intervention model, in the period going from 2007 to 2011 (Period 1), 112 incidents were reported in the Emergency Department (17.2% of the total incidents reported in the hospital [650]), which corresponded to a mean 22.4 incidents per year.
During Period 1, 76 patient identification incidents (68%), 32 medication incidents (28%) and 4 falls (3.5%) were reported in the emergency department.
The medication incidents reported during Period 1 involved errors in dosage (53.1%), drug indication (21.9%) and routes of administration (3.1%). Most errors were made at the time of prescription (59.4%). The drugs most frequently involved were bronchodilators (40.6%), antibiotics (15.6%), electrolytes (9.4%) and antipyretics (6.3%). The errors were detected before they reached the patient in 72% of the cases (this information had not been documented for 6.3%).
In Period 2, the median age of the patients for all incidents reported was 2.6 years (IQR, 1–6 years), and there was a predominance of male patients (52.8%).
The source of the incident report information was the electronic chart in 85.8% of the cases, the patient or a family member in 7.5%, a review of the equipment and/or materials in 5.7%, and the nursing staff in 0.9%.
The type of provider involved in the reported incidents was a physician in 94.3% of the cases compared to a nurse in 5.7%, while the incidents were reported by nurses most often (66%), as well as by nurse assistants (2.8%), doctors (30.2%) and other staff (0.9%).
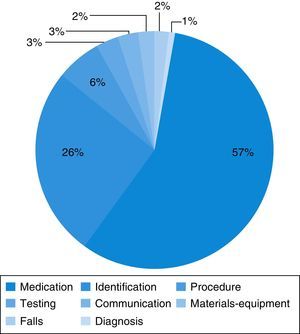
Medication errors were the most frequent type of incident reported (56.6%) (Fig. 2). These incidents most frequently involved errors in dosing (40.6%) and in the route of administration (7.5%), and less frequently errors in the choice of dosage form (3.8%), the regimen (1.8%) or the name of the drug (1.9%), and failure to administer the drug (0.9%). The drugs most frequently involved were bronchodilators (27.4%), antibiotics (5.7%), corticosteroids and intravenous fluids and electrolytes (3.8%).
When it came to severity of the incident, it was low in most cases (75 [70.8%]); moderate in 26 cases (24.5%) and high in 5 (4.7%). The five severe incidents were:
- 1.
Prescription of 350mg of intravenous metasedin to a patient weighing 17kg for the treatment, when needed, of headache, instead of metamizole (similar name); the error did not reach the patient because the patient did not need analgesia and it was detected.
- 2.
Prescription of 8mg of nebulised salbutamol to an infant weighing 8.6kg; the error did not reach the patient because it was detected before the administration of the drug.
- 3.
Error in the diagnosis of a myocarditis due to incorrect assessment of tachycardia in a patient with an underlying metabolic disorder.
- 4.
Prescription of 0.5mg/kg of adrenaline to an infant weighing 4kg suffering from bronchiolitis to be delivered intramuscularly instead of in nebulised form. The error was detected prior to administration and did not reach the patient.
- 5.
Prescription of 200g, as opposed to 200mg, of metamizole to a patient weighing 10kg for treatment of moderate pain. The error was detected before administration and did not reach the patient.
A risk matrix was created for each incident (the product of its frequency and its severity), and the risk to the patient was low in 81.1% of cases, moderate in 12.3% and high in 6.6%.
The incident reached the patient in 38.7% of the cases and the error was detected in time in 61.3% of the cases.
The most frequently identified contributory factors (following the NPSA classification)11 were: individual factors (70.7%), training factors (22.6%), working condition factors (15.1%), task factors (11.3%) and communication factors (9.4%).
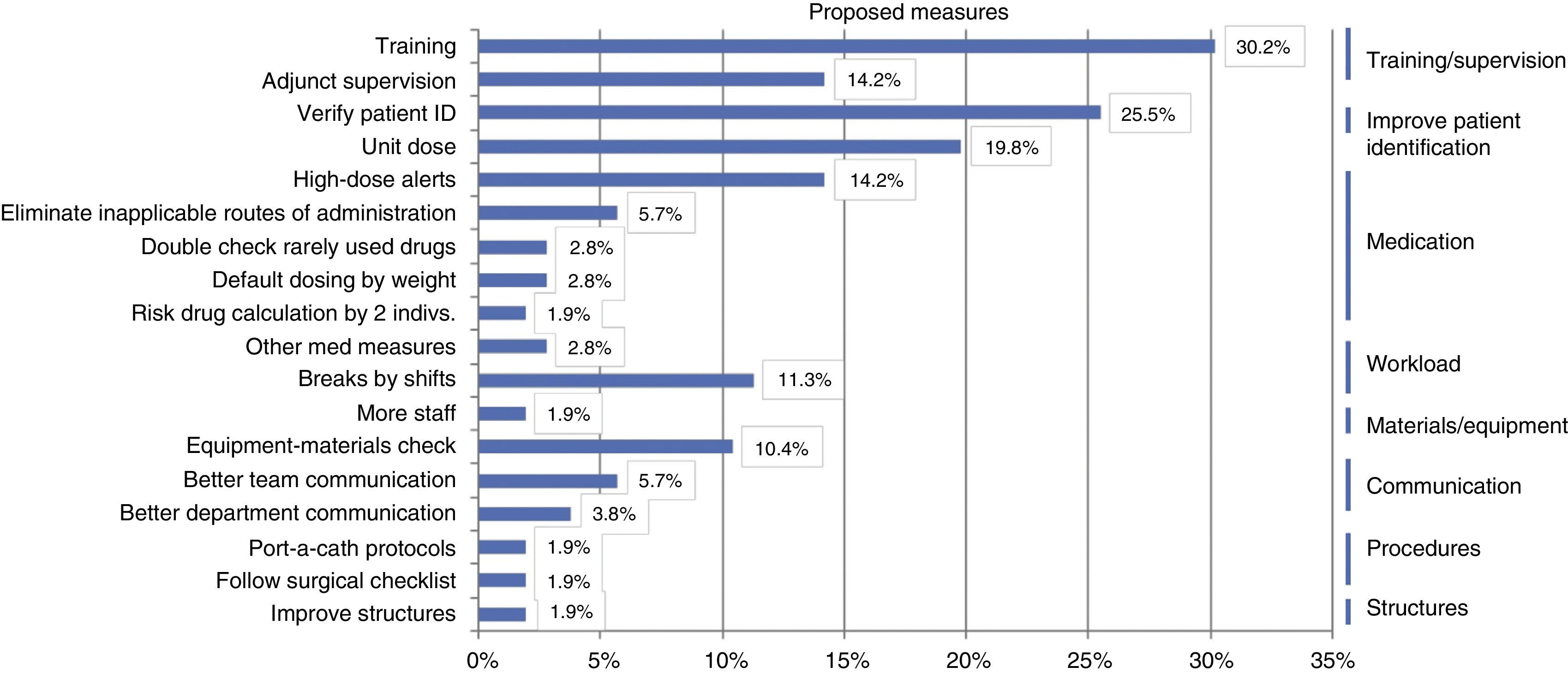
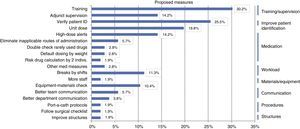
Based on the identified root causes, improvement strategies were proposed, chief of which were: training and supervision measures (54.5%); measures to reduce medication errors, such as the use of unit doses and high-dose alerts (50%); and reinforcing the verification of patient identification (25.5%) (Fig. 3).
We go on to highlight the most important improvement measures adopted in our department:
- –
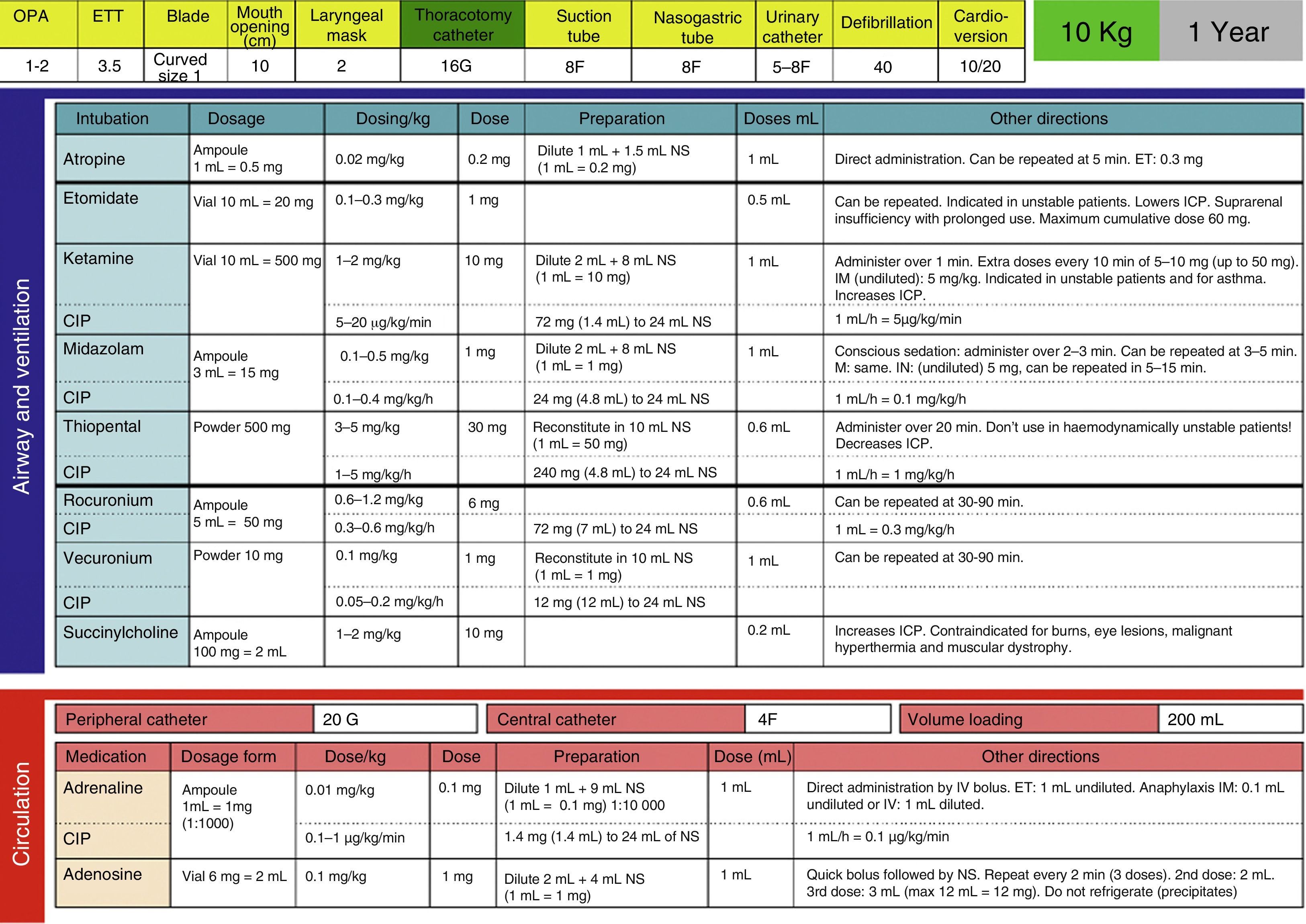
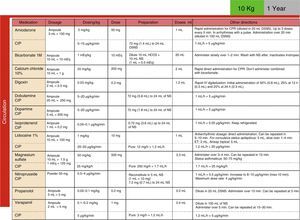
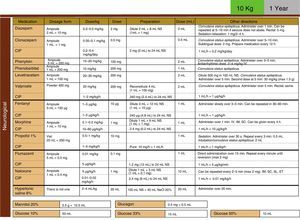
Development, dissemination and use in the resuscitation room of tables for the drugs employed in cardiopulmonary resuscitation (CPR) (Fig. 4).
- –
Implementation of the use of salbutamol unit doses in patients weighing more than 10kg: a unit dose of 2.5mg for patients with weights between 10 and 20kg, and a unit dose of 5mg in patients weighing more than 20kg.
- –
Creation of a surgical checklist to be used in every surgical intervention.
- –
Creation of a summary of the most commonly used emergency care protocols for family physicians, which includes simple protocols for the pathologies most frequently managed in the emergency department.
- –
Setup of alerts for patients that cause particular concern (because they require hourly supervision) in the form of red stickers on the nursing charts.
The development of a new model for the standardised reporting and analysis of incidents facilitated a five-fold increase in incident reporting. Other studies in the literature show that similar systems have had similar beneficial effects.15,16
In light of a low rate of incident reporting, we decided to study the issue. We noticed that the forms were not user-friendly, that their use was not promoted in the various departments of the hospital, and that when report data were collected, there was no subsequent analysis of the causes, they had little impact on improving the system, and feedback was limited to a summary of the reports submitted during each period.
We believe that the implantation and promotion of the new model in the department, emphasising the importance of reporting safety incidents, and providing feedback on the reports and the improvement strategies that have been developed and implemented as a result, is one of the main reasons of the observed increase in reporting. Feedback is particularly important because it promotes the perception that there is a purpose in reporting.10 The development of checklists and new protocols has also contributed to this shift. The introduction of a new reporting form that is anonymous and confidential may also have contributed to the increase in reporting.
The reporting rate has remained similar across the entire hospital before and after the implementation of the new model, probably because the model has also been introduced in other departments and because some of the physicians that work in the emergency department (residents, family physicians, etc.), especially those which have been subject to the improvement interventions in the department, also work in other departments of the hospital.
The incidents mostly involved physicians, but it was the nurses that made the most reports, probably because physicians are afraid of the possible repercussions of reporting in their work environment. On the other hand, most incidents had to do with medical practice, which means that there is no culture of assessing what may require changing in nursing practices, an area in need of intervention and improvement.
In our department, the type of incident reported most frequently was medication errors, as has been observed in other studies.17 Bronchodilators were the agents most commonly involved, most frequently in dosing errors. As a consequence, the use of unit doses of salbutamol was proposed and implemented, which seems to have reduced dosing errors in the administration of this drug. We are currently assessing the impact of this intervention in the reduction of salbutamol dosing errors.
We have also introduced the use of tables of CPR drugs by weight to reduce mistakes in high-stress situations, as is the case of resuscitations, similar to what was observed in a study of patients requiring CPR during ambulance transport, in which there was an improvement in errors in adrenalin administration by using the Broselow dosing charts.18
Other measures described in the literature that have facilitated a reduction in medication-related incidents were the use of barcodes in the administration of opioids,19 automated weight-based dosing calculation for the most frequently used medications,20 improvement of medication reconciliation21 or hanging posters in emergency room cubicles featuring the drugs most commonly involved in dosing incidents with their corresponding dosages in order to avoid prescription errors.22
Another area that has improved in our department is supervision, especially of the least experienced doctors, first-year residents and family physicians, developing specific protocols for their use at the beginning of their emergency medicine rotation.
Other strategies reported in the literature as improving patient safety are a reduction in the weekly hours of work of physicians23 and improvements in provider-family communication.24
The most commonly reported incidents following medication errors were patient identification errors, as reported in other studies,7 so measures have been implemented to reduce them, such as placing the cubicle number across the computer or a periodical reminder of the importance of repeatedly verifying the identify of the patient prior to any procedure or administration of medication.
Most of the reported incidents were low-severity, but their reporting and analysis is important, as it has allowed us to develop many improvement strategies that have helped better the system.9
Incidents were most frequently caused by individual staff factors (physical, psychological, social, personal), lack of experience, fatigue, and so on, which are also the most frequently reported factors in other studies.25,26 Still, the root cause probably does not lie with the individual, but in structural flaws or obstacles to their prevention that have been set by the department (such as lack of training, supervision, or night-time rest).
The analysis of these causes has led to the development of various strategies, for instance in training and in patient identification, aimed at improving the prevention of the most common incidents.
One study has demonstrated the usefulness of nonclinical skills in reducing the incidents caused by individual factors, which, as we have mentioned above, are the most frequent contributory factors.27
Our study allowed us to implement multiple strategies, improving patient safety in the department; other measures are still being developed, such as the creation of posters and messages to be displayed in waiting room screens encouraging patients to inform providers of any allergies or medications they are taking and ask any questions they may have about their diagnosis, procedures or medication; standardising and improving patient transfers; improving the electronic prescription system (alerts, doses by weight, etc.) and the creation of a checklist for patients that are intoxicated, that have underlying metabolic disorders, etc.
This study has the limitations involved in using data collected from voluntary reporting, which can therefore not be used to assess the frequency of the incidents that occur; reporting systems are not used for this purpose, but rather as a means to detect problems. Another limitation is that voluntary reporting is tied to the relevance that the reporter assigns to the event in his or her memory, and there may be biases in the reporting of specific events.
Another useful system to detect incidents is the use of “triggers”, red flags that alert to the possibility that an adverse event has occurred; they are proving useful in the identification of incidents and facilitating their detection.28
Voluntary reporting systems are very helpful in identifying incidents and their causes, but the ideal approach is the combined use of various systems to detect incidents: voluntary reporting, analysis of critical incidents, retrospective chart reviews and modal analysis of failures and events, among others, to obtain a more thorough understanding of the causes.29,30
ConclusionsThe diffusion of the new model in the department, underscoring the importance of reporting incidents, has fostered an increase in reporting. Feedback by means of monthly sessions on the reports received and the improvement measures subsequently developed have promoted the view that reporting is useful and a safety climate in the department.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Vilà de Muga M, Serrano Llop A, Rifé Escudero E, Jabalera Contreras M, Luaces Cubells C. Impacto de un modelo estandarizado para la declaración y análisis de incidentes en la mejora de un servicio de Urgencias pediátrico. An Pediatr (Barc). 2015;83:248–256.
Previous presentation: This study was presented at the XVIII Reunión Anual de la Sociedad Española de Urgencias de Pediatría, 2013. Seguridad en Urgencias. ¿Para qué sirve declarar incidentes?