The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) annually publishes the immunisation schedule which, in our opinion, is considered optimal for children resident in Spain, taking into account the evidence available on current vaccines. Pneumococcal and varicella immunisation in early childhood is already included in all funded vaccines present in the regional immunisation programmes. Furthermore, this committee establishes recommendations on vaccines not included in official calendars (non-funded immunisations), such as rotavirus, meningococcal B, and meningococcal ACWY.
As regards funded immunisations, 2+1 strategy (2, 4, 11–12 months) with hexavalent (DTaP-IPV-Hib-HB) and 13-valent pneumococcal vaccines is recommended.
Administration of the 6-year booster dose with DTaP is recommended, as well as a poliomyelitis dose for children who had received the 2+1 scheme, with the Tdap vaccine for adolescents and pregnant women between 27 and 32 weeks gestation. The two-dose scheme should be used for MMR (12 months and 2–4 years) and varicella (15 months and 2–4 years).
Coverage of human papillomavirus vaccination in girls aged 12 with a two-dose scheme (0, 6 months) should be improved. Information and recommendations for male adolescents about potential beneficial effects of the tetravalent HPV vaccine should also be provided.
ACWY meningococcal vaccine is the optimal choice in adolescents.
For recommended unfunded immunisations, the CAV-AEP recommends the administration of meningococcal B vaccine, due to the current availability in Spanish community pharmacies, with a 3+1 scheme. CAV-AEP requests the incorporation of this vaccine in the funded unified schedule. Vaccination against rotavirus is recommended in all infants.
El Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP) publica anualmente el calendario de vacunaciones que estima idóneo para los niños residentes en España, teniendo en cuenta la evidencia disponible sobre vacunas. Se recogen las vacunas sistemáticas de los calendarios oficiales actuales, con las del neumococo y la varicela en la primera infancia, ya incluidas en todos ellos. Además, este comité realiza recomendaciones sobre vacunas no incluidas en los calendarios oficiales (no financiadas), como rotavirus, meningococo B y meningococo tetravalente.
En cuanto a las vacunas financiadas, se sigue recomendando emplear esquemas 2+1 (2, 4 y 11-12 meses) con las vacunas hexavalentes y con la antineumocócica conjugada 13-valente.
Se aconseja un refuerzo a los 6 años, preferentemente con DTPa, junto a una dosis de polio para aquellos que recibieron esquemas 2+1, así como vacunación con Tdpa en adolescentes y durante el embarazo entre las semanas 27 y 32.
Se emplearán esquemas de 2dosis para triple vírica (12 meses y 2-4 años) y varicela (15 meses y 2-4 años).
Se deben incrementar las coberturas frente al papilomavirus en niñas de 12 años con 2dosis (0-6 meses), así como informar a los varones de los beneficios potenciales de la vacunación y valorar la recomendación del preparado tetravalente.
En adolescentes, la opción óptima es la vacuna antimeningocócica tetravalente.
Respecto a las vacunas recomendadas no financiadas, dada su disponibilidad en las farmacias comunitarias, se recomienda la vacuna frente al meningococo B, con esquema 3+1, y se solicita su entrada en el calendario. Es recomendable vacunar a todos los lactantes frente al rotavirus.
The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) annually updates its immunisation schedule taking into account the current evidence with the purpose of issuing the recommendations for immunisation considered most appropriate for children residing in Spain.
This year, as can be seen in Fig. 1, the main changes introduced by this Committee in the recommendations of the previous year are maintained, and only minor updates have been made. We recommend reading the expanded review of these recommendations at www.vacunasaep.org. On the other hand, the recommendations for special situations and risk groups have been excluded from this document, and can be consulted at the web page referred above.
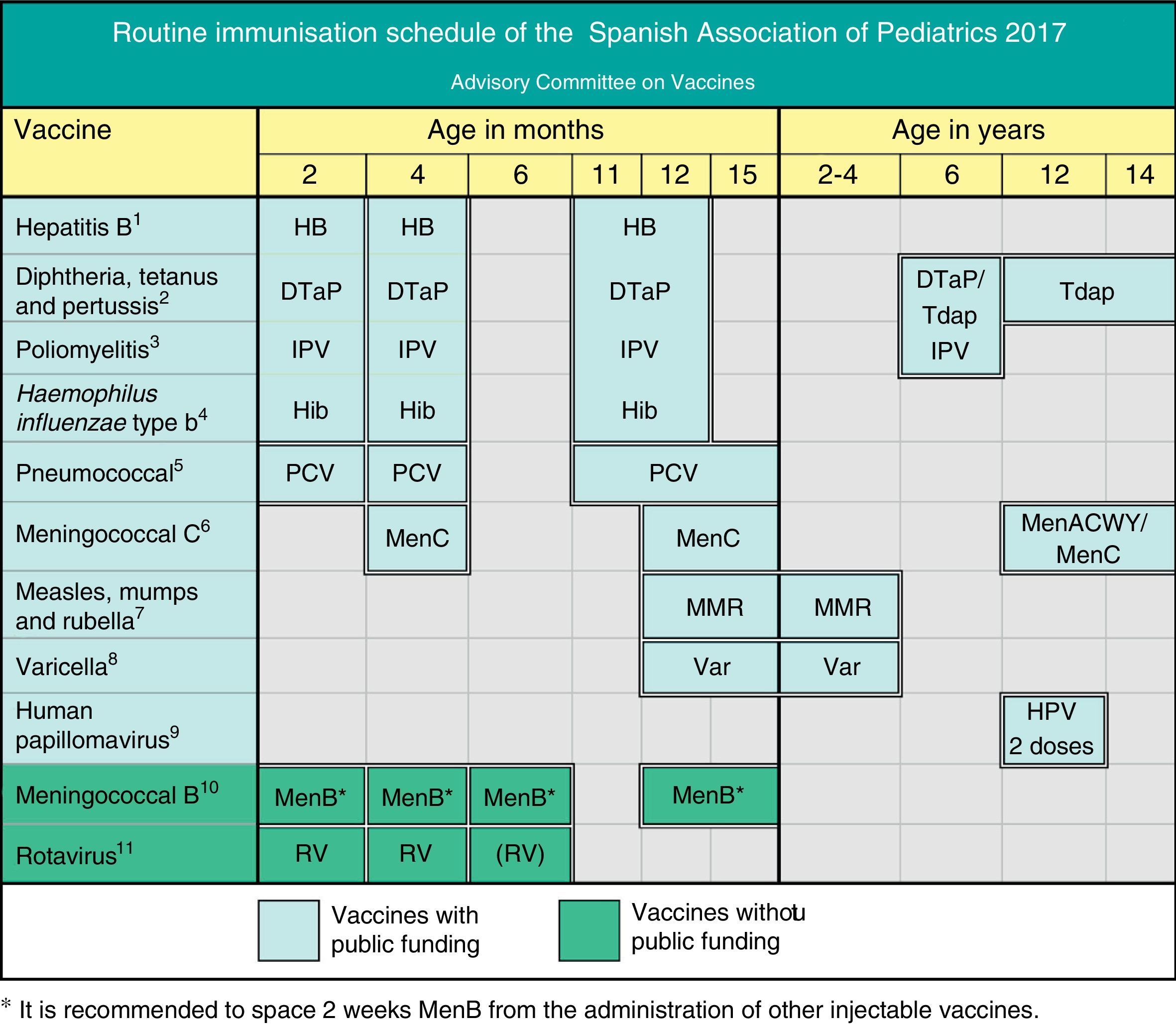
Routine immunisation schedule of the Spanish Association of Paediatrics. 2017 Recommendations. This immunisation schedule, designed for childhood and adolescence, specifies the ages recommended for the administration of the vaccines considered by the CAV-AEP to have the profile of a routine vaccine—vaccines that every child in Spain should receive. It includes the official publicly funded routine vaccines, which are offered for free in every autonomous community, and non-funded routine vaccines that the CAV-AEP recommends be given to all children, but that are not currently publicly funded. The recommended accelerated or catch-up schedules should be applied whenever vaccination is not performed at the specified ages. We recommend consulting the immunisation schedule of the corresponding autonomous community or city. Adverse reactions must be reported to the health authorities.
(1) Hepatitis B vaccine (HB) – three doses of hexavalent vaccine at ages 2, 4 and 11–12 months. Children of HBsAg-positive mothers will also be given one dose of monovalent HB vaccine at birth along with 0.5mL of hepatitis B immune globulin (HBIG) within 12h of birth. When maternal serological status is unknown, the neonatal dose should be administered and maternal serology tested immediately, and should the mother test be positive, HBIG should be administered to the neonate as soon as possible, and in the first week of life. The administration of four doses of HB vaccine is generally acceptable and recommended in children of HBsAg-positive mothers vaccinated at birth and with birth weights of less than 2000g, as the neonatal dose should not be counted in these cases. Unvaccinated children and adolescents should be given three doses of monovalent vaccine or the combined hepatitis A and B vaccine on a 0, 1 and 6 month schedule, at any age.
(2) Diphtheria, tetanus and acellular pertussis vaccine (DTaP/Tdap) – five doses: primary vaccination with two doses, at 2 and 4 months, of DTaP (hexavalent) vaccine; booster at 11–12 months (third dose) with DTaP (hexavalent) vaccine; at 6 years (fourth dose) with the standard load vaccine (DTaP-IPV), preferable to the low diphtheria and pertussis antigen load (Tdap-IPV), and at 12–14 years (fifth dose) with Tdap.
(3) Inactivated poliovirus vaccine (IPV) – four doses: primary vaccination with two doses, at 2 and 4 months, and booster doses at 11–12 months and 6 years.
(4) Haemophilus influenzae type b conjugate vaccine (Hib) – three doses: primary vaccination at 2 and 4 months and booster at 11–12 months.
(5) Pneumococcal conjugate vaccine (PCV) – three doses: the first two at 2 and 4 months with a booster at 11–12 months of age. If routine childhood immunisation is not yet publicly funded, the 3+1 schedule applies: three doses in the first year (2, 4 and 6 months) and a fourth dose at age 12 months. The vaccine currently recommended in Spain is the PCV13.
(6) Meningococcal C conjugate vaccine (MenC) – three doses of monovalent conjugate vaccine given in a 1(2)+1+1 schedule: one dose at age 4 months, another at 12 months, and a final dose at 11–12 years. Depending on the vaccine used, primary vaccination may require one dose (at 4 months) or two (at 2 and 4 months of age). At present, only Madrid applies the two-dose schedule in the first year, at 2 and 4 months. Ideally, the last dose at 12 years will be replaced by one dose of MenACWY, which can be given between age 12 and 14 years.
(7) Measles, mumps and rubella vaccine (MMR) – two doses of measles, mumps and rubella vaccine. The first at 12 months and the second at 2–4 years of age, preferably at 2 years. Susceptible patients outside those age ranges will be vaccinated with two doses at least one month apart.
(8) Varicella vaccine (Var) – two doses: the first at age 15 months (12 months is also acceptable) and the second at 2–4 years of age, preferably at 2 years. Susceptible patients outside those age ranges will be vaccinated with two doses at least one month apart.
(9) Human papillomavirus vaccine (HPV) – only for females, although families with male children should be informed of the possibility of administering the HPV vaccine, preferably HPV4, since the HPV2 vaccine has been authorised but there is practically no experience of its use in males. Administer two doses at 11–12 years. The schedule is based on the vaccine used: for the tetravalent vaccine, a two-dose series (0 and 6 months) in females aged 9 to 13 years and a three-dose series (0, 2 and 6 months) in females aged 14 or more years; and for the bivalent vaccine, a two-dose series (0 and 6 months) in females aged 9 and 14 years and a three-dose series (0, 1 and 6 months) in females aged 15 or more years. It can be administered at the same time as the MenC, hepatitis A and B and Tdap vaccines. There are no data on its simultaneous administration with the varicella vaccine.
(10) Meningococcal B vaccine (MenB) – four doses: the first three in the first year of life (at 2, 4 and 6 months) with a booster at 12–15 months of age, although it is recommended that it be given 15 days apart from other injectable vaccines to minimise its potential reactogenicity, and simultaneous vaccination with MenC should be avoided.
(11) Rotavirus vaccine (RV) – two or three doses of rotavirus vaccine: at 2 and 4 months (Rotarix®) or at 2, 4 and 6 months (RotaTeq®). It is very important that primary vaccination be initiated between 6 and 12 weeks from birth to minimise risk, and vaccination should be completed by 24 weeks (Rotarix®) or 32 weeks (RotaTeq®). Doses must be administered at least four weeks apart. Both doses may be given at the same time as any other vaccine. *It is recommended that the MenB vaccine be administered two weeks apart from other injectable vaccines.
The AEP rejoices in the decision of the Ministry of Health to include vaccination against varicella and pneumococcus in the immunisation schedules of the autonomous communities (ACs),1 as it will prevent a large number of cases of these diseases and their complications. It is also important that the varicella and meningococcal B vaccines, as well as the two commercially distributed rotavirus vaccines, be available in community pharmacies.
Due to the short supply of the pertussis vaccine, and for the purpose of optimising the immunisation schedule, adapting it to current epidemiological conditions, increasing its efficacy and converging towards the homogenisation of vaccination schedules in Europe, the CAV-AEP has decided to maintain the 2+1 schedule with hexavalent vaccines.
Ideally, scientific societies would be taken into account in the decision-making process, and the autonomous communities (ACs) and the Ministry of Health would make a greater collective effort to fund a more comprehensive routine immunisation schedule. Alternative systems should be set up to assist families in paying for vaccines that are not funded by the state, as is done for commonly used medications.
In order to prevent the re-emergence of vaccine-preventable diseases, we need to continue vaccinating all children, striving to maintain high coverage rates and to persuade parents that refuse vaccination.
Vaccination against hepatitis B2017 recommendation:we recommend the vaccination of infants with three doses of hexavalent vaccine at 2, 4 and 12 months of age. Four-dose schedules may be administered in infants that received a first dose at birth. Previously unvaccinated older children and adolescents will receive 3 doses of the monovalent vaccine alone or combined with the hepatitis A vaccine at 0, 1 and 6 months.
In Spain, the annual incidence of hepatitis B (HB) remains at fewer than two cases per 100000 inhabitants (estimated incidence in 2015, 1.47).2
In 2016, in half of the ACs, the first dose of the vaccine was administered at birth, a schedule that should be maintained considering that control of HB during pregnancy is suboptimal. Vaccination of newborns is always to be performed with the monovalent vaccine, which will be prescribed to children born to mothers that are HBsAg-positive or of unknown serological status. Babies born to HBsAg-positive mothers should also be given anti-hepatitis B immune globulin (HBIG), preferably in the first 12h of life.
Vaccination against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus influenzae type B2017 recommendation:we recommend primary vaccination with the hexavalent DTaP-IPV-Hib vaccine at 2 and 4 months. The first dose can be given earlier at 6 weeks post birth. A booster dose, also of the hexavalent vaccine, should be administered at 11 to 12 months of age (2+1 schedule), with a subsequent booster with the DTaP-IPV, preferably, and otherwise the Tdap-IPV vaccine at 6 years, and another booster dose with Tdap vaccine at age 12 to 14 years. Vaccination with a dose of Tdap is recommended in all pregnant women starting at 27 weeks’ gestation, preferably before 32 weeks, and in all household members that will be in contact with the newborn whenever possible (with emphasis on vaccination of mothers that were not vaccinated during pregnancy in the immediate postpartum period).
The incidence of pertussis has increased worldwide. Infants suffer the most severe and lethal forms of disease. Preventive strategies must prioritise the protection of this group. Vaccination of pregnant women with the Tdap vaccine is safe and efficacious,3 and is the most effective and efficient method for preventing pertussis in infants. Vaccination during the second trimester of gestation produces higher levels of antibodies in newborns and increases the opportunity for vaccination,4 and thus countries such as the United Kingdom (UK) recommend its administration starting at 20 weeks’ gestation.5
The 2+1 schedule is used in many European countries and has been recommended by the Interterritorial Council of the Spanish National Health Service (Consejo Interterritorial del Sistema Nacional de Salud [CISNS]) for 2017.1 This schedule, which is safe and immunogenic, optimises the use of the available doses.
The DTaP vaccine is preferred over low-antigen load formulations (Tdap) for the booster at age 6 years, because it confers a longer-lasting protection.6
Children that received a 2+1 primary vaccination series with hexavalent vaccine as infants must receive a polio booster at age 6 years, preferably with the DTaP-IPV vaccine. The booster dose at age 6 years with the Tdap vaccine remains suspended for the time being due to the short supply of the vaccine. Since vaccine coverage rates are high and these children have already received four doses, we do not expect this to have a negative impact.
Vaccination against pneumococcal disease2017 recommendation:vaccination against pneumococcal disease is recommended for all children younger than 5 years and children that are immunocompromised or otherwise at risk at any age, with administration of a 2+1 series in infants (2, 4 and 11–15 months). The CAV-AEP reasserts that the 13-valent pneumococcal conjugate vaccine (PCV13) is most appropriate for routine vaccination in Spain, as it provides the best coverage for the currently circulating serotypes in the country, and thus can have the greatest impact on the control of pneumococcal disease.
In the past year, a substantial amount of data was published regarding the impact of pneumococcal conjugate vaccines—10-valent (PCV10) and 13-valent (PCV13)—on invasive pneumococcal disease (IPD). Both are very effective against the pneumococcal serotypes that they contain7–13 and can provide indirect protection (herd immunity) against vaccine serotypes in unvaccinated individuals,9–13 so their ultimate impact depends to a great extent on the prevalence of these serotypes in the geographical area where the vaccines are used. The use of the PCV10 could be appropriate when the there is little to no circulation of serotypes 19A, 6A and 3. In the Autonomous Community of Madrid, IPD due to serotype 19A has been nearly eradicated in the paediatric age group following routine vaccination of children with the PCV13, but 19A continues to be the third most frequent serotype involved in IPD in adults (5% of cases) following serotypes 8 (15.6%) and 3 (8.6%).14 In the Autonomous Community of Valencia, serotypes 3 and 19A account for 16.8% and 5%, respectively, of cases of IPD in which the serotype was identified (when it came to serotype distribution, they were the first and third most prevalent).15
Generally speaking, both vaccines lead to an expansion or emergence of non-vaccine serotypes, which may partially reduce their overall impact on IPD.10–12
The current evidence shows that the PCV10 does not induce herd immunity against serotypes 19A and 6A. While it provides significant cross-protection against these serotypes, this only occurs in vaccinated children and does not seem to last more than one or two years. In fact, data in Finland has shown that the increased incidence of cases caused by serotypes 3 and 19A in unvaccinated individuals ultimately cancels out the impact of the PCV10 in the overall IPD burden, despite the reduction in cases caused by vaccine serotypes.11
Both vaccines reduce the incidence of noninvasive pneumococcal disease, leading to a decreased rate of hospitalization due to pneumonia, both pneumococcal and of unknown aetiology.16,17
The effectiveness of PCVs against acute otitis media (AOM) is greater than the one expected based on efficacy studies. It is well known that a first episode of AOM in infants disturbs local defence mechanisms, predisposing the individual to future episodes. A recent study showed that vaccination with the PCV13 reduces not only the incidence of pneumococcal AOM caused by vaccine serotypes, but also the episodes of AOM caused by other bacteria.18
Vaccination against group C meningococcal disease2017 recommendation: we recommend three to four doses of the monovalent meningococcal conjugate vaccine (MenC) (1+1+1 or 2+1+1 series). The recommended schedule is: 4 months, 12 months, and 12 years of age. Depending on the vaccine used, primary vaccination may require two doses at 2 and 4 months, or a single dose at 4 months. In adolescents, an ideal alternative would be the use of the tetravalent meningococcal vaccine (ACWY).
Extensive evidence on the effectiveness of the monovalent vaccine against MenC has been accrued over the years.19 The incidence of group C invasive meningococcal disease (IMD) in Spain decreases each year, and remained very low in the 2014–2015 season (0.04 cases/100000 inhabitants).20 In this season, 28 cases of IMD caused by serotypes other than B and C have been reported (0.06 cases/100000 inhabitants), three of which were caused by serogroup Y and four by serogroup W. In the first five months of 2016, there has been an increase in the isolation of serotype W, with the number of isolates doubling compared to 2015, and if this trend continues, by the end of the year the number of isolations may be five times that of previous years.21 Tetravalent conjugate vaccines (Menveo® and Nimenrix®),22 which are reserved to travellers to endemic areas, constitute an optimal option for the booster dose in adolescents (12–14 years), as frequency of travelling increases starting at this age.
In the UK, the MenC dose at 3 months of age was eliminated in 2016, maintaining the doses at ages 12 months and 14 years. This decision was based on the success of the vaccination campaign started in 1999, which has led to the near eradication of paediatric cases of group C IMD. Children obtain indirect protection from universal vaccination of infants at 12 months and of adolescences (with the tetravalent vaccine in the latter group), and the introduction in the childhood immunisation programme of the MenB vaccine, which could confer a degree of protection against MenC.23
Vaccination against measles, mumps and rubella (MMR)2017 recommendation:we recommend that a first dose of MMR vaccine is given at 12 months of age, with a second dose given between 2 and 4 years of age, preferably at 2, for the early remediation of any potential primary vaccine failures.
Following the trend of recent years, the number of measles cases notified in the WHO European Region has decreased, but not as much as expected, causing deaths and severe complications. There are also still cases of rubella and outbreaks of mumps. All of the above results mainly from the low vaccination coverage in several countries. In Spain, the incidence is low, although the same trends have been observed.24 It is essential to maintain high vaccination coverage and rigorous epidemiological surveillance in order to eradicate these diseases. A single dose of vaccine at age 12 months achieves seroconversion rates of 95% and higher for all three viruses, and the rates approximate 100% after the second dose.
Vaccination against varicella2017 recommendation:it is recommended that all children are vaccinated against varicella with two doses: one at 15 months and another at 2–4 years of age. It is also recommended that a two-dose catch up vaccination be done in all children and adolescents that have not had the disease and are unvaccinated (or that the 2-dose series is completed if applicable).
In 2016, all the ACs incorporated vaccination against varicella with a series of two doses (at ages 15 months and 3–4 years).1,25 The two available vaccines (Varilrix® and Varivax®) have been shown to be highly effective (96.8% in Navarre, 92% in the USA) in reducing the incidence of varicella and its complications in both the vaccinated and the unvaccinated population,26,27 and exhibit an excellent safety profile.28 In the 20 years of vaccination in the United States, there has been evidence of a sustained reduction in the incidence of disease.29
There has been no evidence of an age shift in the onset of varicella or changes in the incidence of herpes zoster (HZ). Childhood vaccination against varicella could be considered cost-effective if it were known that it does not cause an increase in the incidence of HZ in the general population, especially in individuals aged more than 50 years, while we await evidence on the potential impact of vaccination against HZ in this age group.28 In this regard, it is important to ensure the two doses in adolescents, administering catch-up doses as needed.
Vaccination against human papillomavirus (HPV)2017 CAV-AEP recommendation:routine vaccination against HPV is recommended for all girls, preferably at age 12 years, as a means to prevent cervical and anal cancer and precancerous lesions in the female genital tract. Health professionals should provide information and consider recommending the tetravalent vaccine to male patients.
The optimal age for vaccination is 12 years with a 2-dose series (Fig. 1). Thus, administration before sexual debut provides the maximum potential benefits of this vaccination. The recommendation also extends to older ages if vaccination has been delayed for different reasons, given the benefits that it may continue to provide.
This vaccination is showing a high efficacy and effectiveness in universal vaccination programmes in the prevention of persistent HPV infection, genital warts and precancerous cervical lesions, preventing up to 85% of high-grade dysplasias.30 In a few years, data will probably be available on the actual prevention of cervical cancer and other types of cancer associated with HPV.
With more than 200 million doses having been administered worldwide, these vaccines have proven to be safe and to have a very favourable risk–benefit ratio.30,31 Research has not found an association between these vaccines and the development of autoimmune and neurologic diseases.31,32 Still, the average coverage in Spain does not exceed 80%. Health professionals must reinforce the positive messages regarding this vaccine to improve its acceptance in the population.
The tetravalent vaccine, authorised for use in males, is included in the immunisation schedules of the United States, Australia, Canada, Austria, Switzerland and some regions in Italy. There are relevant data on the role of HPV in the aetiology and pathogenesis of certain cancers affecting both sexes, but especially those with a higher incidence in males, such as anal cancer and cancers of the head and neck. Furthermore, there has been no evidence in Europe of indirect protection of males resulting from vaccination programmes that target female adolescents.33 Thus, health professionals should provide information and consider recommending the tetravalent vaccine to male patients, preferably at age 12 years. Cervarix has been authorised for use in males, but at present there is little experience on its use.
The upcoming introduction of a 9-valent HPV type 6/11/16/18/31/33/45/52/58 vaccine that has already been authorised by the EMA is expected to increase prevention of HPV-related cervical cancers from 70% to 90%, and may prevent 85% to 95% of HPV-related vulvar, vaginal and anal cancers.34
Vaccination against group B meningococcal disease2017 recommendation: the meningococcal B vaccine exhibits the profile of a routine vaccine to be administered to all children starting at 2 months of age.
Clinical trials of the only vaccine authorised in Europe for the prevention of group B IMD (MenB, Bexsero®) starting at age 2 months have demonstrated that the vaccine is immunogenic and safe and that it induces immunological memory. The trials conducted this far show that it can be given at the same time as the other vaccines in the immunisation schedule, including the MenC vaccine, although this could increase reactogenicity. The use of prophylactic paracetamol reduces the incidence of adverse effects without compromising the immunogenicity of the vaccine or of any other of the vaccines included in the immunisation schedule that are given at the same time, but its routine use is not recommended when the vaccine is administered alone.
In September 2015, the UK included this vaccine in its childhood immunisation programme, starting in infants with a 2+1 series (at ages 2, 4 and 12–13 months).35 The results documented in the first 10 months of the programme have been reported, showing a coverage of 88% for the first two doses.36 The effectiveness has been estimated at 83% against any meningitis B strain and 94% against vaccine preventable strains. Reported cases of IMD due to MenB have dropped by 50% in the vaccine-eligible population.36 At any rate, the total number of cases is very low, and these results will probably change with time.
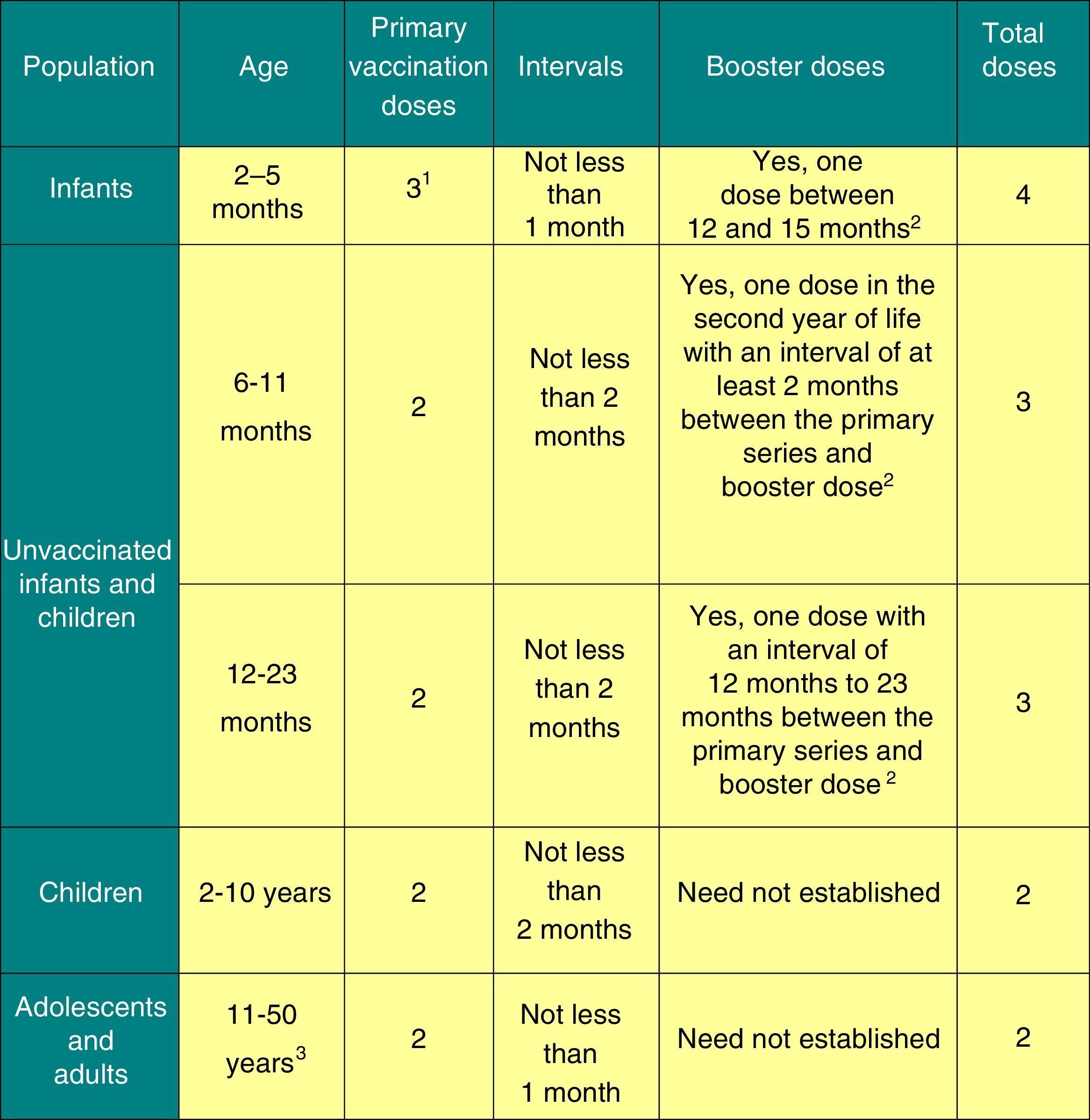
The dosage of this vaccine is presented in Fig. 2.
Posology of the meningococcal B vaccine.
1 The first dose should be given starting at 2 months of age. The safety and efficacy of 4CMenB in infants less than 8 weeks of age has not yet been established.
2 The need and intervals for other booster doses have yet to be established.
3 There are no data in adults aged more than 50 years.
2017 recommendation:vaccination against rotavirus is an advisable health intervention for all infants.
Year 2016 has marked the tenth anniversary of the introduction of this vaccine. By May 2016, 81 countries had introduced it in their official immunisation schedules, and the resulting health benefits have been monumental, with a significant reduction in the morbidity and mortality associated with gastroenteritis due to rotavirus (RV) in infants and young children in low- and high-income countries.
In neighbouring countries with routine immunisation against rotavirus, such as the United Kingdom (UK), a reduction of 87% of the episodes has been observed relative to the ten preceding seasons.37
The benefits that derive from vaccination against rotavirus, be they direct ones in vaccinated infants or indirect ones in contacts due to herd immunity, are very clear.38 The evidence on the extraintestinal spread of RVs and their involvement in systemic diseases (especially neurologic) continues to grow.39
The benefits of vaccination against rotavirus continue to significantly outweigh the hypothetical risks of intussusception, the only serious adverse effect associated with it. Postmarketing surveillance studies in developed countries with routine vaccination against rotavirus have demonstrated that episodes of intussusception may result from vaccination, but the risk is low (1–5 cases per 100000 vaccinated children).40
In June 2016, the AEMPS lifted the suspension of marketing authorization of the Rotarix® vaccine, which had been held since 2010 in Spain, so the two vaccines against RV are now available in pharmacies.
Both can be administered at the same time as other vaccines, and their dosage can be consulted in Fig. 1.
FundingThe development of these recommendations (analysis of the published data, debate, consensus and publication) has not been supported by any funding source outside of the logistic support provided by the AEP.
Conflicts of interestIn the last 5 years:
DMP has collaborated in educational activities funded by Astra, Pfizer and Sanofi Pasteur MSD, and as consultant on a GlaxoSmithKline advisory board.
FJAG has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD and as a consultant on GlaxoSmithKline and Novartis advisory boards.
JAF has collaborated in educational activities and as a researcher in clinical trials funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a consultant on Novartis, GlaxoSmithKline and Astra Zeneca advisory boards.
MJCO has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials conducted by Pfizer, and as a consultant on GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD advisory boards.
JMCR has collaborated in educational activities funded by GlaxoSmithKline, Sanofi Pasteur MSD and Novartis.
NGS has collaborated in educational activities funded by Sanofi Pasteur MSD, Novartis and Pfizer, and attended educational activities funded by Novartis and Pfizer.
AHM has received funding to attend domestic educational activities.
THSM has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials conducted by GlaxoSmithKline and Pfizer.
MMM has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials conducted by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a consultant on a Novartis advisory board.
LOC has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials conducted by GlaxoSmithKline.
JRC has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials conducted by GlaxoSmithKline and Pfizer.
- -
David Moreno-Pérez. Paediatric Infectious Diseases and Immunodeficiencies. Paediatrics Clinical Management Unit, Hospital Materno-Infantil, Hospital Regional Universitario de Málaga. IBIMA Research Group. Department of Paediatrics and Pharmacology, School of Medicine, Universidad de Málaga.
- -
Francisco José Álvarez García. Paediatrician. Centro de Salud de Llanera, Asturias. Adjunct Professor of Health Sciences, Department of Medicine, Universidad de Oviedo.
- -
Javier Arístegui Fernández. Unit of Paediatric Infectious Diseases, Hospital Universitario de Basurto, Bilbao. Department of Paediatrics, School of Medicine, Universidad del País Vasco (UPV/EHU).
- -
María José Cilleruelo Ortega. Department of Paediatrics, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid. Department of Paediatrics, School of Medicine, Universidad Autónoma de Madrid.
- -
José María Corretger Rauet. Advisory Council on Vaccines, Department of Health, Generalitat de Catalunya, Barcelona.
- -
Nuria García Sánchez. Paediatrician. Centro de Salud Delicias Sur. Zaragoza. Adjunct Professor of Health Sciences, Department of Paediatrics, School of Medicine, Universidad de Zaragoza.
- -
Ángel Hernández Merino. Paediatrician. Centro de Salud La Rivota, Alcorcón, Madrid.
- -
Teresa Hernández-Sampelayo Matos. Department of Paediatrics, Hospital General Universitario Gregorio Marañón. Department of Paediatrics, School of Medicine, Universidad Complutense de Madrid.
- -
Manuel Merino Moína. Paediatrician. Centro de Salud El Greco, Getafe, Madrid. Lecturer, School of Medicine, Universidad Europea, Madrid.
- -
Luis Ortigosa del Castillo. Department of Paediatrics, Hospital Universitario Nuestra Señora de Candelaria. Department of Paediatrics, School of Medicine, Universidad de La Laguna, Santa Cruz de Tenerife.
- -
Jesús Ruiz-Contreras. Department of Paediatrics, Hospital Universitario 12 de Octubre, Madrid. Department of Paediatrics, School of Medicine, Universidad Complutense de Madrid.
Please cite this article as: Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al. Calendario de vacunaciones de la Asociación Española de Pediatría (CAV-AEP): recomendaciones 2017. An Pediatr (Barc). 2017;86:98.e1–98.e9.
The members of the Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) are listed in Appendix 1.