The implantation of the neonatal screening programme in Spain since 1980 and the associated improvement in outcomes has resulted in an increase in the number of pregnant women that have phenylketonuria (PKU).1
During pregnancy, the amount of maternal phenylalanine (Phe) that crosses the placenta increases, and can be neurotoxic and teratogenic for a developing foetus with an immature liver.1–6 The abnormalities in the offspring of women with uncontrolled PKU during pregnancy were first reported by Dent in 1957 and Mabry et al. in 1963.2
The term maternal phenylketonuria syndrome (MPKUS) refers to the teratogenic effects of intrauterine exposure to Phe on the developing foetus, both physical and cognitive.1,2 Teratogenicity is minimal to non-existent if maternal levels of Phe are maintained between 120 and 360μmol/L (2–6mg/dL) for the 3 months preceding conception and throughout pregnancy,1–6 although some authors have reported that serum levels of Phe of less than 120μmol/L can also be associated with embryopathy.4,6
We present the case of a patient aged 32 years with classic PKU and mild phenotype with optimal preconceptional metabolic control. The prenatal ultrasound scans were normal and the serum Phe remained under 240μmol/L during pregnancy.
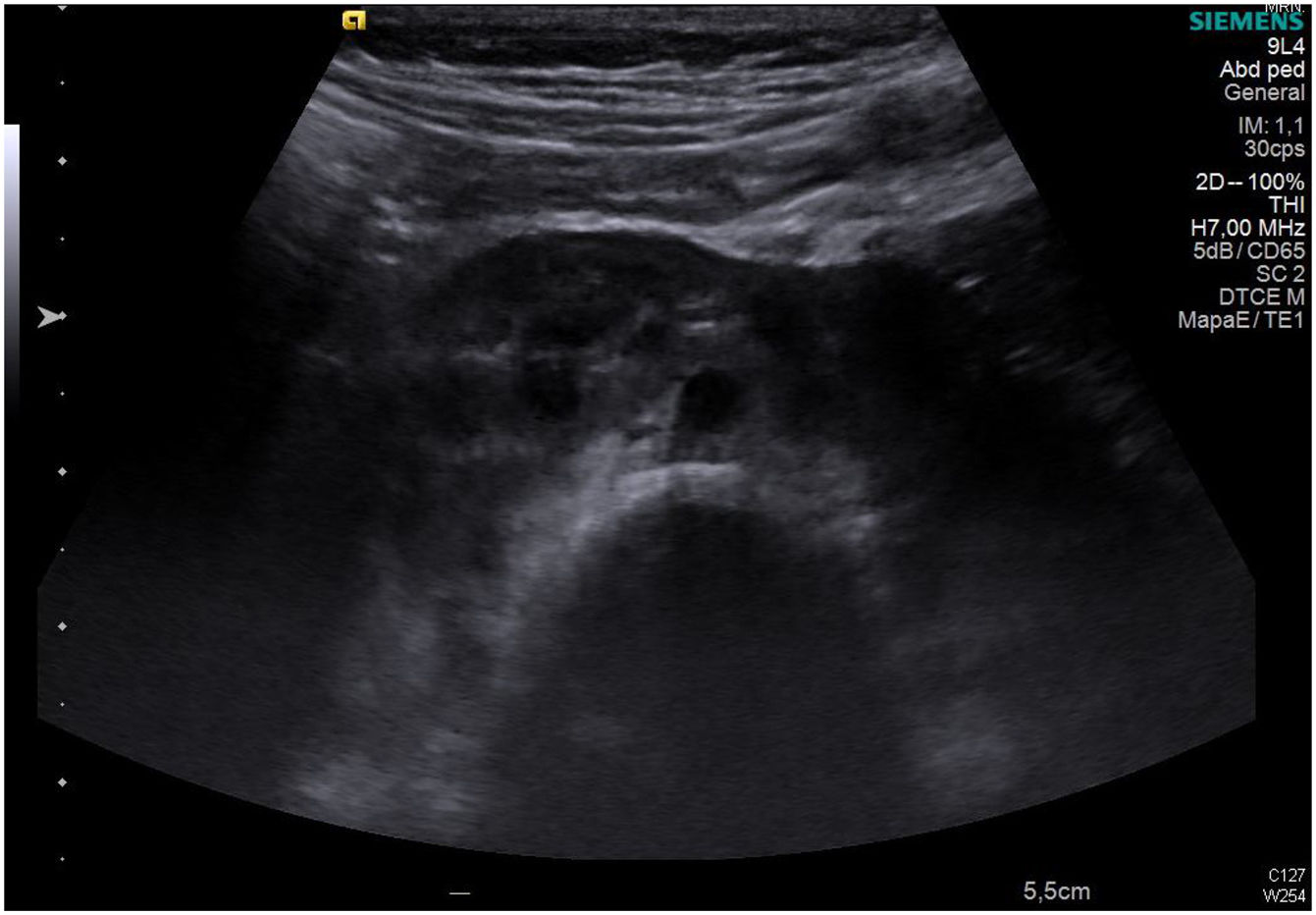
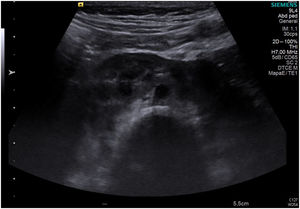
The patient gave birth at term to a newborn with normal phenotype, normal findings in the transfontanellar ultrasound and negative neonatal screening results. At 1 month post birth, an abdominal ultrasound scan detected a horseshoe kidney in the infant (Fig. 1).
Horseshoe kidney is the most frequent renal fusion anomaly and occurs in 0.4–1.6 per 10000 live births. It consists of 2 renal masses oriented vertically on either side or to one side of the midline fused by an isthmus of parenchyma or fibrous tissue that traverses the medial plane. In more than 90% of cases, the kidneys are fused at the distant poles.
Maternal PKU syndrome may manifest with intellectual disability (92% of cases), microcephaly (73%), intrauterine growth restriction (40%), heart disease (12%) and oesophageal atresia and facial malformations.1–6 Gokmen et a were the first to describe the presence of unilateral renal agenesis in an infant with maternal PKU syndrome.3 However, the effects of hyperphenylalaninemia on renal development is yet unknown. The facial dysmorphic features are similar to those found in foetal alcohol syndrome.2
As for the treatment of the pregnant mother with PKU, not all drugs and nutritional supplements available to individuals with PKU are appropriate for use in pregnancy. Thus, supplementation with large neutral amino acids during pregnancy is not recommended, as it does not consistently decrease the serum levels of Phe.2
Sapropterin is a drug that can be used during pregnancy after considering its potential risks and benefits for the mother and the foetus. It is recommended that women that were taking sapropterin before conception continue to do so during pregnancy.1
Maternal Phe requirements do not remain the same throughout pregnancy, as they are lower in the first and second trimester and increase in the third trimester, when the liver of the foetus is more mature. Therefore, the mother must be under strict nutritional control during pregnancy to avoid severe dietary restrictions resulting in insufficient energy and protein intakes.1,2,6
Monitoring of vitamin and mineral intake is recommended.1,2 The foods used for medical treatment in patients with PKU may result in elevation of vitamin A levels, which is associated with congenital defects. There is also evidence linking a deficient intake of vitamin B12 with an increased risk of congenital heart defects.4,5
In the postpartum period, maternal Phe requirements decrease, and strict metabolic and nutritional control continues to be necessary. Breastfeeding is not contraindicated in these mothers, as infants who are not affected by phenylalanine hydroxylase deficiency can easily metabolise the slightly elevated Phe levels in breast milk.
We believe that the case we present here is relevant, as it is the first in a pregnant woman with PKU in our region where despite strict prenatal control the child had a renal anomaly. This may have been a chance occurrence, but it could also be a malformation resulting from maternal hyperphenylalaninemia that has not yet been described in the literature. We should also emphasise the importance of testing pregnant women for PKU in countries where neonatal screening is not universal in order to identify affected women whose children could benefit from dietary treatment of the mother during pregnancy, which can prevent maternal PKU syndrome.1
Please cite this article as: Márquez Armenteros AM, Plácido Paias R. Riñón en herradura: casualidad o embriopatía por fenilcetonuria materna. An Pediatr (Barc). 2019;90:248–249.