Current guidelines in Spain recommend performing transthoracic echocardiography (TTE) in all children under 2 years of age with a heart murmur. In 2014, the American Paediatric Association published the first appropriate use criteria (AUC) for outpatient paediatric transthoracic echocardiography (TTE) to promote its cost-efficient use. The aim of this article is to analyse the AUC and other clinical factors as predictors of congenital heart disease (CHD) in children less than 2 years of age with a heart murmur, and to develop a safe and efficient referral strategy.
Patients and methodCase–control study conducted with children less than 2 years of age, referred from Paediatric Primary Care to Paediatric Cardiology during a 4-year study. A predictive model for CHD was determined using multivariate analysis.
ResultsA total of 688 patients were included, with 129 (19%) cases of CHD. An age less than 3 months (adjusted odds ratio [ORa] 3.8 [1.5–8.4], p=0.030) and fulfilling AUC (ORa 16.3 [9.4–28.3], p<0.001) were predictors of CHD. Concurrent infection (ORa 0.6 [0.2–0.8], p<0.001) and a negative neonatal screening with pulse oximetry (ORa 0.1 [0.05–0.4], p=0.001) decreased the risk of CHD. The referral strategy that included these criteria had a 98% sensitivity, 39% specificity, and positive and negative predictive values of 27% and 99%, respectively. It could not diagnose 2% of CHD (all mild), and showed a 32% TTE reduction rate compared to our current strategy.
ConclusionTo refer children less than 3 months old, fulfilling AUC, without a concurrent infection, or without negative neonatal pulse oximetry screening, is a safe and efficient strategy for the management of heart murmur in children under 2 years of age.
La Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas recomienda realizar ecocardiografía transtorácica (ETT) en todo menor de 2 años con soplo. En 2014 la Asociación Americana de Pediatría publicó los primeros criterios de uso apropiado de ETT pediátrica ambulatoria (CUA) como guía para promover un uso costo-eficiente de la misma. Nuestro objetivo fue analizar los CUA y otros factores clínicos como predictores de cardiopatía congénita (CC) en menores de 2 años con soplo para desarrollar una estrategia de derivación eficiente y segura.
Pacientes y métodoEstudio de casos y controles en menores de 2 años derivados por pediatría de atención primaria a cardiología pediátrica por soplo durante 4 años. Mediante análisis multivariante se determinó un modelo predictivo de CC.
ResultadosSe incluyeron 688 pacientes con 129 casos (19%) de CC. La edad menor a 3 meses (odds ratio ajustada [ORa] 3,8[1,5-8,4]; p=0,030) y cumplimiento de CUA (ORa 16,3[9,4-28,3]; p<0,001) fueron predictores de CC. La presencia de infección concurrente (ORa 0,6 [0,2-0,8]; p<0,001), y un screening neonatal con pulsioximetría negativo (ORa 0,1 [0,05-0,4]; p=0,001) disminuyeron el riesgo de CC. La estrategia de derivación que incluía estos criterios presentó una sensibilidad 98%, especificidad 39%, VPP 27% y VPN 99%. Dejaría de diagnosticar un 2% de CC (todas leves) y reduciría un 32% el número de ETT realizadas respecto a la estrategia actual.
ConclusiónLa derivación por pediatría de atención primaria para ETT de niños que sean menores de 3 meses, cumplan CUA, no presenten infección concurrente, o no tengan SP, constituye una estrategia eficiente y segura para el manejo de soplo en menores de 2 años.
Heart murmurs constitute the most frequent reason for referral from paediatric primary care (PPC) to paediatric cardiology (PC) services.1–3 They are a common finding in the physical examination (prevalence, 5–80%) and may be the initial sign that eventually leads to diagnosis of congenital heart disease (CHD).4,5 Transthoracic echocardiography (TTE) is the gold standard for diagnosis of CHD. It is a safe and accessible method that can be interpreted quickly and with a high diagnostic yield when performed by an expert. The Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Society of Paediatric Cardiology and Congenital Heart Disease [SECPCC]) recommends referring every child aged less than 2 years that has a murmur for a TTE evaluation, even if the patient is asymptomatic and the presentation is suggestive of an innocent murmur.6 This is justified by the need to assess for silent CHD that may go undetected in the clinical assessment, the lack of cooperation of patients in this age group in the physical examination, and for the purpose of preventing family anxiety, unnecessary evaluations and restrictions to the physical activity of the child.
However, TTE is not a cost-effective technique in the management of heart murmurs in the paediatric age group.7–10 In 2014, the American Academy of Paediatrics established the first appropriate use criteria (AUC) for initial TTE in outpatient paediatric cardiology.11 This document, designed to promote the appropriate and efficient use of TTE, includes 113 indications for echocardiography, classifying as appropriate those expected to add information to the initial clinical judgement and where the benefit to the patient exceeds the expected negative consequences (including the economic cost, the risks associated with the procedure and the potential hazard of missed diagnoses). A multicentre study that assessed the implementation of the AUC12 found that heart murmur was the most frequent indication for TTE (30%), and innocent murmur the indication most frequently rated as inappropriate. Furthermore, appropriate referral for the indication of murmur only exhibited good diagnostic accuracy for the detection of CHD (sensitivity, 95%; specificity, 33%; PPV, 46%; NPV, 91%). Thus, the rational and efficient use of TTE for assessment of heart murmurs in the paediatric population based on the application of AUC may provide the foundation for high-quality care, safely reducing the use of TTE for assessment of innocent murmurs.
The aim of our study was to analyse the AUC and other clinical factors that were not among those criteria as predictors of CHD in children aged less than 2 years, and, based on the results of this analysis, to establish a referral strategy and assess its diagnostic accuracy, safety and associated reduction in the use of echocardiography compared to following the current recommendations of the SECPCC.
Patients and methodsDesign, sample and setting of the study: case–control study conducted in the PC unit of a tertiary care hospital between January 2013 and January 2017.
Inclusion criteria: children aged less than 2 years referred for the first time from PPC to the outpatient PC unit for assessment of a heart murmur. All patients underwent evaluation by TTE. We considered patients that received a diagnosis of CHD cases, and further subdivided this group into mild if the patient only required monitoring, moderate if the patient required some type of non-urgent treatment (pharmacotherapy, cardiac catheterization or surgery), and severe if the patient required urgent treatment. In patients with more than one cardiac defect, we used the most severe one for the purpose of classification. We considered patients in whom the findings of the evaluation were normal or incidental (mild peripheral pulmonary stenosis, patent foramen ovale, persistent left superior vena cava, very small ductus or coronary artery fistula in newborns, very mild mitral or tricuspid valve regurgitation, very mild pulmonary valve regurgitation) controls.
Exclusion criteria: referral from setting other than PPC, previous TTE, lack of documentation of relevant variables in the health records.
We obtained the information for this study from the review of health records by a single individual who was an independent observer from outside the PC unit, and who collected data on the following variables: age, sex, history of CHD in first-degree relatives, personal history of factors clearly associated with CHD (malformation syndromes, chromosome disorders, exposure to substances and drugs during gestation, perinatal vertical infection, pregestational diabetes), negative results in neonatal pulse oximetry screening (POS) for heart disease, concurrent infection at the time the murmur was detected at the PPC level, type of murmur detected on auscultation in the PC unit (normal if no murmur detected; innocent if the murmur had the following characteristics: short duration (proto-/mesosystolic), low intensity (1–2/6), soft or sweet, increased in the supine position, absence of additional abnormal sounds; pathologic if the murmur had any of the following characteristics: holosystolic, diastolic or continuous, intensity greater than 3/6, presence of thrills, harsh, abnormal second heart sound, presence of clicks, increased in seated position or after Valsalva manoeuvre), presence of signs or symptoms associated with CHD detected in PC unit (faltering weight, fatigue and sweating during feeding or crying, recurrent respiratory tract infections, central cyanosis detected by pulse oximetry, differences in blood pressure between upper and lower extremities, high blood pressure, tachypnoea (for age), tachycardia (for age), hyperdynamic precordium, thrills, hepatomegaly), type of CHD, treatment required (medication, haemodynamic support or surgery), clinical outcome of CHD (persistence, if CHD persisted at the end of followup; or spontaneous resolution, if the CHD resolved without the need of intervention during the followup), duration of followup, time elapsed to spontaneous resolution of CHD.
Type of referral based on AUC: appropriate referral (indications 40 and 41 of the document; pathologic murmur on auscultation or presumptively innocent murmur on auscultation associated with signs of cardiovascular disease or risk factors for cardiovascular disease in patient or family) and inappropriate referral (indication 39 of the document: absence of murmur or innocent murmur on auscultation with no alarming clinical signs or personal or family risk factors for cardiovascular disease).10
Study protocol: we compared cases and controls to identify potential differences in the variables under study and determined which were independent predictors of CHD. Based on these results, we designed a referral strategy that included these variables and analysed their diagnostic accuracy in detecting CHD, their safety (number and type of CHDs that were not detected, as well as their outcomes), and the reduction in the proportion of echocardiographic evaluations performed compared to the strategy of referring all patients aged less than 2 years.
Statistical analysis: we performed a descriptive analysis, summarising qualitative variables as absolute frequencies and percentages and comparing them in cases and controls by means of the chi square test or the Fisher exact test as applicable. We assessed the association between variables by means of the odds ratio (OR). We summarised quantitative variables as mean and standard deviation (SD) or as median and interquartile range (IQR) based on their distribution; we used the Kolmogorov–Smirnov test to test the assumption of normality, and compared relative frequencies in cases and controls by means of the Student's t test. We then proceeded to fit a multivariate logistic regression model for predicting the detection of CHD. We included in the model variables that were clinically significant or for which we found a statistically significant association in the bivariate analysis. To better fit the model, we used the method of backward stepwise regression. We have expressed the association of CHD with variables that we considered to be independent predictors as adjusted odds ratios (aORs) After fitting the final model for the prediction of CHD, we calculated its sensitivity, specificity and positive and negative predictive values. We defined statistical significance as a p-value of less than 0.05 for a 95% confidence interval (CI). We analysed the data with the software IBM© PASW STATISTICS© version 18.0 (IBM Corporation, Somers, NY, USA).
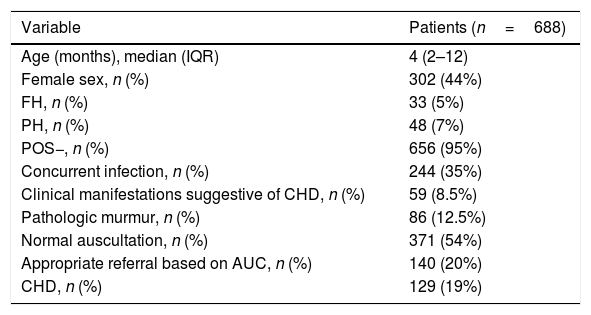
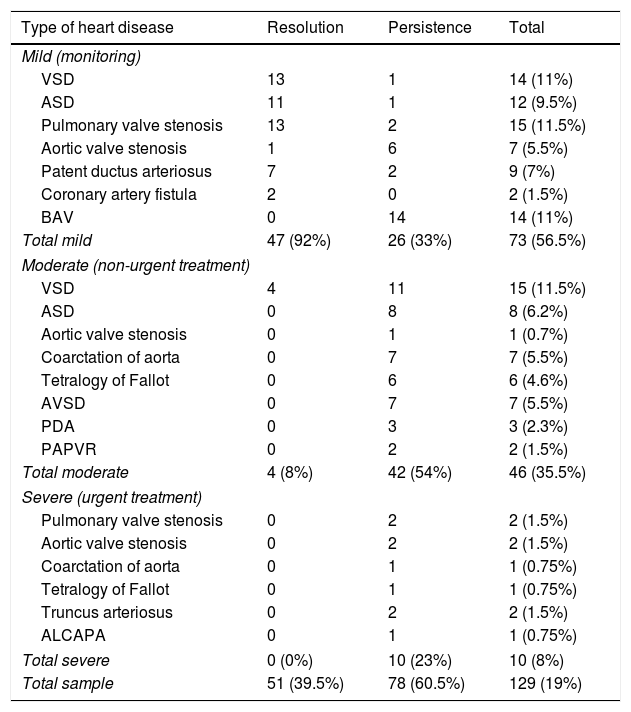
ResultsDescriptive (Tables 1 and 2): we included 688 patients, among whom we identified 129 cases (19%) of CHD (57% mild, 35% moderate, and 8% severe). Spontaneous resolution occurred in 39.5% of the patients with CHD at a mean of 11.3 months (SD, ±6.8), with a mean duration of followup of 3.7 years (SD, ±1.7). The time elapsed between referral from primary care and the appointment at the PC clinic was 0.9 months (SD, ±0.4).
Demographic and clinical characteristics of the sample.
| Variable | Patients (n=688) |
|---|---|
| Age (months), median (IQR) | 4 (2–12) |
| Female sex, n (%) | 302 (44%) |
| FH, n (%) | 33 (5%) |
| PH, n (%) | 48 (7%) |
| POS−, n (%) | 656 (95%) |
| Concurrent infection, n (%) | 244 (35%) |
| Clinical manifestations suggestive of CHD, n (%) | 59 (8.5%) |
| Pathologic murmur, n (%) | 86 (12.5%) |
| Normal auscultation, n (%) | 371 (54%) |
| Appropriate referral based on AUC, n (%) | 140 (20%) |
| CHD, n (%) | 129 (19%) |
AUC, appropriate use criteria for referral of the American Academy of Paediatrics; CHD, congenital heart disease; FH, family history of CHD; IQR, interquartile range; PH, personal history of risk factors for CHD; POS−, neonatal pulse oximetry screening for CHD performed with negative results.
Types of congenital heart disease detected, and their outcomes.
| Type of heart disease | Resolution | Persistence | Total |
|---|---|---|---|
| Mild (monitoring) | |||
| VSD | 13 | 1 | 14 (11%) |
| ASD | 11 | 1 | 12 (9.5%) |
| Pulmonary valve stenosis | 13 | 2 | 15 (11.5%) |
| Aortic valve stenosis | 1 | 6 | 7 (5.5%) |
| Patent ductus arteriosus | 7 | 2 | 9 (7%) |
| Coronary artery fistula | 2 | 0 | 2 (1.5%) |
| BAV | 0 | 14 | 14 (11%) |
| Total mild | 47 (92%) | 26 (33%) | 73 (56.5%) |
| Moderate (non-urgent treatment) | |||
| VSD | 4 | 11 | 15 (11.5%) |
| ASD | 0 | 8 | 8 (6.2%) |
| Aortic valve stenosis | 0 | 1 | 1 (0.7%) |
| Coarctation of aorta | 0 | 7 | 7 (5.5%) |
| Tetralogy of Fallot | 0 | 6 | 6 (4.6%) |
| AVSD | 0 | 7 | 7 (5.5%) |
| PDA | 0 | 3 | 3 (2.3%) |
| PAPVR | 0 | 2 | 2 (1.5%) |
| Total moderate | 4 (8%) | 42 (54%) | 46 (35.5%) |
| Severe (urgent treatment) | |||
| Pulmonary valve stenosis | 0 | 2 | 2 (1.5%) |
| Aortic valve stenosis | 0 | 2 | 2 (1.5%) |
| Coarctation of aorta | 0 | 1 | 1 (0.75%) |
| Tetralogy of Fallot | 0 | 1 | 1 (0.75%) |
| Truncus arteriosus | 0 | 2 | 2 (1.5%) |
| ALCAPA | 0 | 1 | 1 (0.75%) |
| Total severe | 0 (0%) | 10 (23%) | 10 (8%) |
| Total sample | 51 (39.5%) | 78 (60.5%) | 129 (19%) |
ALCAPA, anomalous left coronary artery from the pulmonary artery; ASD, atrial septal defect; AVSD, atrioventricular septal defect; BAV, bicuspid aortic valve; PDA, patent ductus arteriosus; PAPVR, partial anomalous pulmonary venous return; VSD, ventricular septal defect.
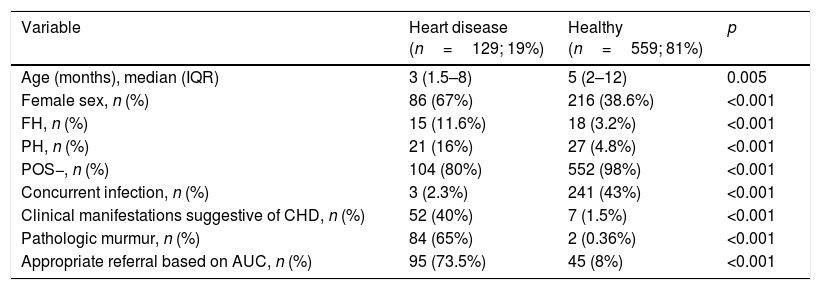
Predictors of congenital heart disease: we found statistically significant differences in the following variables: age, female sex, family history, personal history, negative POS, concurrent infection, pathologic murmur, cardiovascular symptoms and type of referral based on AUC (Table 3).
Comparison of variables under study in cases and controls.
| Variable | Heart disease (n=129; 19%) | Healthy (n=559; 81%) | p |
|---|---|---|---|
| Age (months), median (IQR) | 3 (1.5–8) | 5 (2–12) | 0.005 |
| Female sex, n (%) | 86 (67%) | 216 (38.6%) | <0.001 |
| FH, n (%) | 15 (11.6%) | 18 (3.2%) | <0.001 |
| PH, n (%) | 21 (16%) | 27 (4.8%) | <0.001 |
| POS−, n (%) | 104 (80%) | 552 (98%) | <0.001 |
| Concurrent infection, n (%) | 3 (2.3%) | 241 (43%) | <0.001 |
| Clinical manifestations suggestive of CHD, n (%) | 52 (40%) | 7 (1.5%) | <0.001 |
| Pathologic murmur, n (%) | 84 (65%) | 2 (0.36%) | <0.001 |
| Appropriate referral based on AUC, n (%) | 95 (73.5%) | 45 (8%) | <0.001 |
AUC, appropriate use criteria for referral of the American Academy of Paediatrics; CHD, congenital heart disease; FH, family history of CHD; IQR, interquartile range; PH, personal history of risk factors for CHD; POS−, neonatal pulse oximetry screening for CHD performed with negative results.
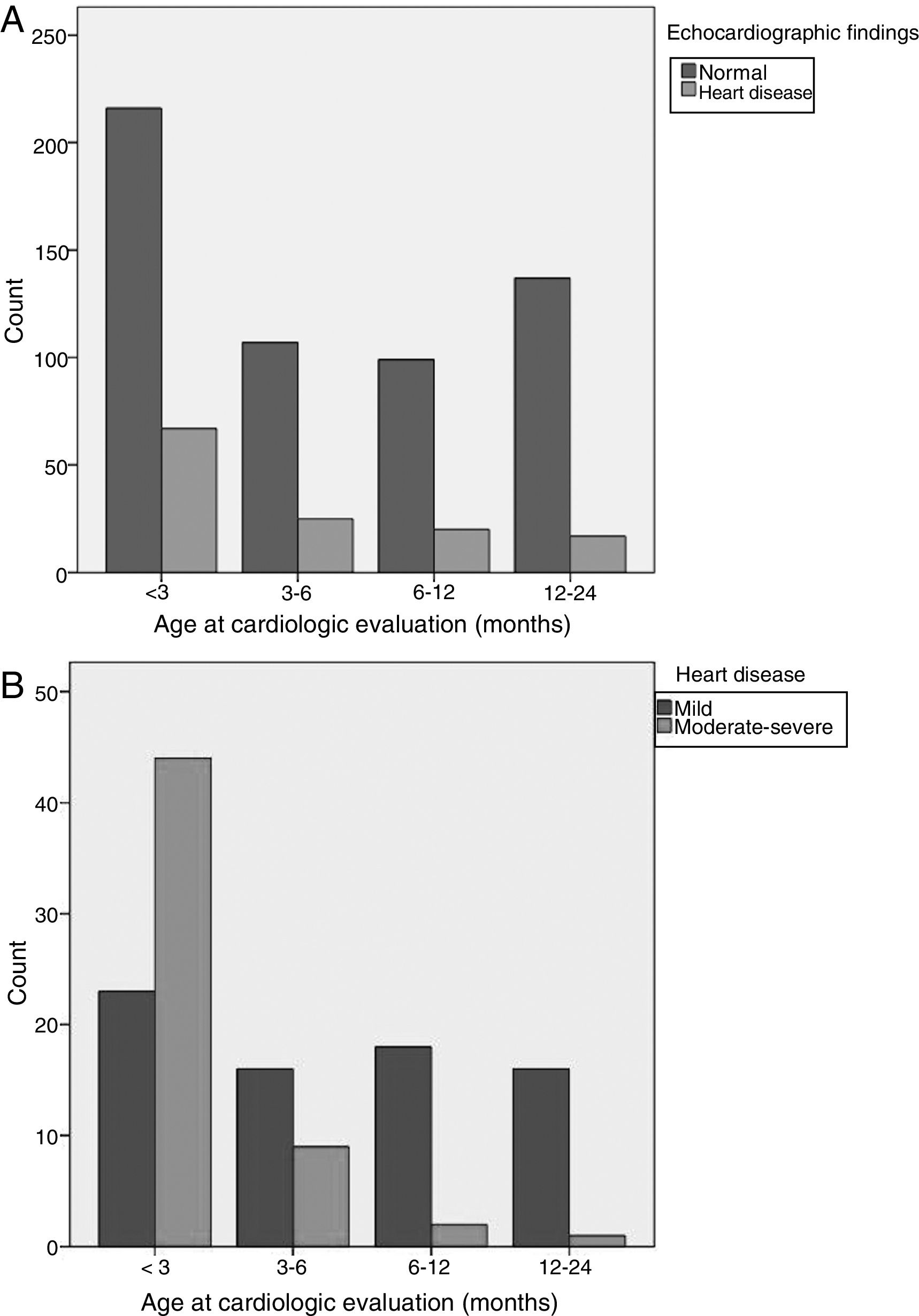
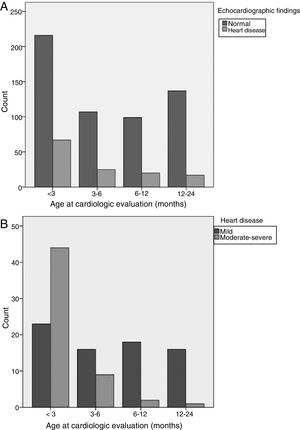
Age (Fig. 1): infants aged less than 3 months were at greater risk of CHD. Fifty-two percent of all cases of CHD, 78% of cases of moderate CHD and 80% of cases of severe CHD occurred in this age group. The 12–24 months and 6–12 months age intervals were associated with a lower risk of CHD. Ninety-two percent of cases detected in children aged more than 6 months were mild.
Variables included in the AUC: appropriate referral was associated with an increased risk of detection of a CHD. Sixty-eight percent of patients that had been referred appropriately had a CHD, including all patients with severe CHD and 93% of patients with moderate CHD. All the variables used to define appropriate referral were significantly associated with an increased risk of CHD, and pathologic murmur and clinical manifestations suggestive of cardiovascular disease were the strongest predictors for a CHD diagnosis.
Variables not included in the AUC: a negative POS was associated with a lower risk of CHD. We found severe CHD detectable by screening in 19% of patients without a negative POS result. Concurrent infection was associated with a lower risk of CHD. Twenty-two percent of patients with no concurrent infection had a CHD, and all CHDs detected in the group of patients with concurrent infection were mild.
Table 4 presents the results of the bivariate and multivariate analyses performed in the study.
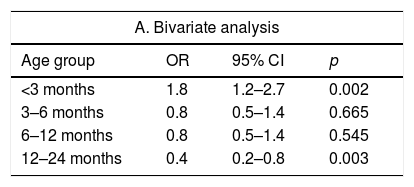
Analysis of the risk of having a CHD by age group, AUC variables and non-AUC variables.
| A. Bivariate analysis | |||
|---|---|---|---|
| Age group | OR | 95% CI | p |
| <3 months | 1.8 | 1.2–2.7 | 0.002 |
| 3–6 months | 0.8 | 0.5–1.4 | 0.665 |
| 6–12 months | 0.8 | 0.5–1.4 | 0.545 |
| 12–24 months | 0.4 | 0.2–0.8 | 0.003 |
| Appropriate use criteria variables | OR | 95% CI | p |
|---|---|---|---|
| Pathologic murmur | 519.8 | 123.8–2182.8 | <0.001 |
| Manifestations suggestive of CHD | 53.2 | 23.3–121.4 | <0.001 |
| PH | 3.8 | 2–7 | <0.001 |
| FH | 3.9 | 1.9–8 | <0.001 |
| Appropriate referral based on AUC | 31.9 | 19.4–52.4 | <0.001 |
| Non-AUC variables | OR | 95% CI | p |
|---|---|---|---|
| POS– | 0.5 | 0.2–0.7 | <0.001 |
| Concurrent infection | 0.3 | 0.01–0.9 | <0.001 |
| B. Multivariate analysis | |||
|---|---|---|---|
| Variable | aOR | 95% CI | p |
| Age <3 months | 3.8 | 1.5–8.4 | 0.030 |
| Age 12–24 months | 0.6 | 0.3–2.5 | 0.402 |
| Concurrent infection | 0.6 | 0.2–0.8 | <0.001 |
| POS− | 0.1 | 0.05–0.4 | 0.001 |
| Appropriate referral based on AUC | 16.3 | 9.4–28.3 | <0.001 |
aOR, adjusted odds ratio (the reference group for calculation of aORs was the group of patients aged 3–12 months); AUC, appropriate use criteria for referral of the American Academy of Paediatrics; CHD, congenital heart disease; FH, family history of CHD; PH, personal history of risk factors for CHD; POS–, neonatal pulse oximetry screening for CHD performed with negative results.
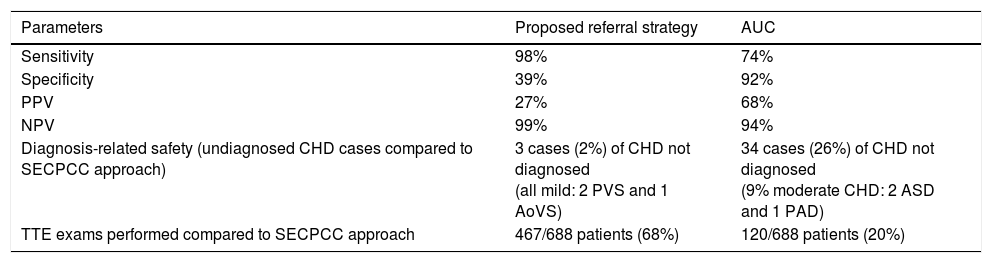
Alternative referral strategy: based on the results of the multivariate analysis, we defined our alternative referral strategy as performance of TTE only in patients meeting at least one of the following criteria: appropriate referral based on the AUC, age less than 3 months, absence of negative POS result, or detection of murmur in the absence of concurrent infection. This strategy had a sensitivity of 98%, a specificity of 40%, a PPV of 27% and a NPV of 99% for detection of CHD. With the alternative referral strategy, 2% of the CHDs detected with the current strategy would have been missed, all of them corresponding to cases of mild CHD that had favourable outcomes during the followup. The alternative referral strategy that we devised would have included 468 patients and avoided performance of 220 TTE examinations (a reduction of 32% compared to applying the recommendations of the SECPCC). Table 5 compares the diagnostic accuracy, safety and difference in use of TTE using the selected strategy and the AUC compared to applying the recommendations of the SECPCC.
Comparison of the proposed referral strategy and the AUC.
| Parameters | Proposed referral strategy | AUC |
|---|---|---|
| Sensitivity | 98% | 74% |
| Specificity | 39% | 92% |
| PPV | 27% | 68% |
| NPV | 99% | 94% |
| Diagnosis-related safety (undiagnosed CHD cases compared to SECPCC approach) | 3 cases (2%) of CHD not diagnosed (all mild: 2 PVS and 1 AoVS) | 34 cases (26%) of CHD not diagnosed (9% moderate CHD: 2 ASD and 1 PAD) |
| TTE exams performed compared to SECPCC approach | 467/688 patients (68%) | 120/688 patients (20%) |
AoVS, aortic valve stenosis; ASD, atrial septal defect; AUC, appropriate use criteria for outpatient paediatric echocardiography; CHD, congenital heart disease; PDA, patent ductus arteriosus; PVS, pulmonary valve stenosis; TTE, transthoracic echocardiography; SECPCC, Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas.
The excessive use and high cost of cardiac imaging techniques compared to other elements of medical care call for the identification of strategies that are safe and have a high yield for the assessment of highly prevalent problems, such as paediatric heart murmurs.9,13,14 The findings of our study suggest that the key would be the prioritisation of TTE based on the clinical evaluation of the primary care paediatrician, with particular consideration of the AUC proposed by the American Academy of Paediatrics, the age of the patient, the performance and results of POS and the presence or absence of concurrent infection.
Most children referred to PC for a heart murmur do not have a CHD. In agreement with the previous literature,9,10,15 the diagnostic yield of TTE for CHD found in our study was low (19%), and referrals had only been appropriate in 20% of the sample, which was mainly due to the high proportion of inappropriate referrals for assessment of innocent murmurs. Routine TTE evaluation of all patients with a heart murmur involves significant costs. Yi et al.7 demonstrated that universal referral for TTE involved an incremental cost of $158,000 per additional detected case of CHD of any severity. In our case, with 129 CHDs detected during the period under study (many of which were mild and resolved spontaneously), this would amount to a cost of 20,382,000 euro (5,095,500 euro/year). Furthermore, the indiscriminate use of TTE may be counterproductive, as it can lead to incidental findings that will prompt unnecessary tests or increase parental anxiety, which persists in 7–10% of cases.10,16,17 Several studies suggest that clinical assessment by a paediatric cardiologist could be an adequate strategy to select patients with heart murmur eligible for TTE.7,8,13 But TTE is accessible, safe and has a high diagnostic yield, which, combined with the anxiety of the family and their expectation that it will be performed, and with the increase in lawsuits for medical errors, makes it difficult to avoid performing it once the patient enters PC services, even if the murmur appears to be innocent. Thus, referral to PC for assessment of a heart murmur invariably involves performance of TTE, at least in Spain, and the detection of CHD in children aged less than 2 years with heart murmurs would be more efficient if some type of screening were performed, which, in our opinion, would depend on the rational use of referral to PC by primary care paediatricians.
Determining which heart murmurs require evaluation by TTE is a diagnostic challenge for primary care paediatricians,18,19 who, due to a diverse set of circumstances (lack of clinical guidelines, limited skills and experience when it comes to heart murmurs, excessive workloads, concerns regarding liability, family anxiety, high availability of outpatient TTE, etc.) tend to have a low threshold for referral.18 In relation to this, several studies1,20 have found that patients referred for assessment of murmurs account for an increasing amount of the workload in PC settings, with referrals increasing by up to 20% every 5 years. There is also evidence of a decreasing diagnostic yield of TTE for assessment of heart murmurs in the past few decades, from 50% to 16%, which, combined with the stable incidence of CHDs (<1%), suggests that there have been changes in the approach to the management of heart murmurs in PPC, with an increase in the frequency of referral of patients with innocent murmurs.1,10,20
The AUC were published to guide clinical decision-making in children with possible heart disease.11 Despite its adequate yield in the detection of CHDs,12 our results suggest that the exclusive use of TTE, given its moderate sensitivity (78%), is not an adequate screening strategy for significant diseases such as CHD. This is our opinion in light of the high prevalence of CHD found in our study, mainly corresponding to silent lesions in patients in whom the findings of the physical examination were normal. Of all the variables considered in the AUC, the detection of a pathologic murmur on auscultation in PC and the presence of clinical manifestations suggestive of cardiovascular disease corresponded to the highest probability of detection of a CHD. This is consistent with the results of the study that assessed the outcomes of implementing the AUC,12 which found that indication 41 (pathologic murmur on auscultation) had the highest yield for detection of CHDs (40%). Although the features of an innocent murmur on auscultation have been clearly defined,3,21 accurate assessment depends to a great extent on the clinical skills, experience and self-competence of primary care paediatricians. We did not analyse the diagnostic yield of auscultation at the PPC level, but several studies have shown that it is insufficient,22,23 so it would be important to improve the teaching of auscultation skills to paediatricians in training.24,25 For the above reasons, while the AUC may be effective in detecting most moderate and severe CHDs, it could be prudent to include additional criteria to help primary care paediatricians correctly stratify children aged less than 2 years with heart murmurs.
Age is an essential factor in the management of heart murmurs. Most CHDs are diagnosed at young ages, which is mainly due to the increase in early diagnosis associated with the use of antenatal and neonatal echocardiography,26 POS27–29 and the multiple routine health checkups performed during childhood. In addition, most out-of-hospital deaths due to undiagnosed CHDs occur in infants aged less than 12 weeks, and most severe CHDs become symptomatic within 1 year from birth.30,31 Therefore, the strategies for the management of heart murmur must be adapted to the patient's age, with a lower threshold for referral at younger ages.3,10,14,15,26 In our study, age less than 3 months was an independent predictor of CHD in patients with heart murmur. Furthermore, the risk of CHD was lower in patients aged more than 6 months, so it does not seem necessary to refer all children aged less than 2 years that have a murmur to rule out CHD.
The use of neonatal screening for heart disease by means of pulse oximetry has an adequate diagnostic yield for detection of severe CHD.27,28,32,33 The diagnosis of these CHDs is delayed in up to 30% of cases when screening is not performed. Our findings were similar to those in the previous literature, as severe CHDs were detected in 19% of the patients without a negative POS, while a negative POS was associated with a lower risk of having a CHD. Since at present neonatal POS for heart disease is not performed routinely, this is an important factor to take into account in the evaluation of these patients.
Heart murmurs are a very frequent occurrence in the context of concurrent infection in children aged less than 2 years (35% in our sample), and concurrent infection was independently associated with a lower risk of CHD. We ought to note that the murmur disappeared in 54% of these patients when the infection resolved. Thus, the presence of a concurrent infection suggests that a CHD is unlikely.4 Since the mean wait time to assessment by PC was of 1 month, the results of our study suggest that it may be advisable to wait at least this time before referring the patient for TTE.
LimitationsThe main limitations of this study were its retrospective design and the fact that the information collected by the independent observer was based solely on the patient health records of the PC unit. This may be a significant limitation when it comes to including variables in a predictive model to assess strategies of referral from PPC to PC, as assessments may differ between PPC and PC settings. Nevertheless, variables such as a negative POS result and the personal and family history tend to remain stable through time, the presence of concurrent infection and the clinical manifestations of CHD were always documented at the time of detection of murmur at the PPC level, and the physical examination (and auscultation in particular) is the gold standard for assessment of a paediatric heart murmur, and therefore the method that should be used, ideally, at the PPC level. Due to all of the above, we consider that the data recorded in the PC unit can be extrapolated to the information that should be obtained at the PPC level in the assessment of heart murmurs, so that this limitation would only be significant if the aim of the study had been to compare the diagnostic accuracy of assessment at the PPC level versus the PC level.
ConclusionsThe combined use of AUC and the additional factors proposed in our study appeared to be a safe and efficient strategy that would reduce the number of TTE examinations performed for assessment of heart murmurs in children aged less than 2 years compared to following the current recommendations of the SECPCC.
Educational interventions based on these results may help optimise the use of TTE for assessment of murmurs at the PPC level.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodríguez-González M, Alonso-Ojembarrena A, Castellano-Martínez A, Estepa-Pedregosa L, Benavente-Fernández I, Lubián López SP. Soplo cardíaco en menores de 2 años: buscando una estrategia de derivación eficiente y segura. An Pediatr (Barc). 2018;89:286–293.
Previous presentation: This study has been presented in the 65 Congress of the Asociación Española de Pediatría; June 1–3, 2017; Santiago de Compostela, Spain.