Anti-tissue transglutaminase antibodies (tTG) have high specificity for coeliac disease (CD). However, positive anti-tTG antibodies have been described in non-coeliac patients.
AimTo assess positive anti-tTG antibodies not related to gluten intake.
Patients and methodsRetrospective review and follow up conducted on patients with suspected CD (increase anti-tTG levels and gastrointestinal symptoms) but with atypical serology results, positive anti-tTG with gluten free diet and a decrease in anti-tTG levels despite gluten intake.
ResultsA total of 9 cases were reviewed in which 5 cases had Marsh 3 involvement in the initial biopsy, and were diagnosed with CD (Group A). They began a gluten free diet and also a cow's milk protein (CMP) free diet because of their nutritional status. When CMP was re-introduced, anti-tTG increased, and returned to normal after the CMP was withdrawn again. The other 4 patients had a normal initial biopsy (Group B). Gluten was not removed from their diet, but they started a CMP free diet because a non IgE mediated CMP allergy was suspected. Symptoms disappeared, and anti-tTG was normal after CMP free diet with gluten intake. All the patients had susceptibility haplotype HLA DQ2/DQ8.
ConclusionsCMP ingestion after an exclusion diet can induce an increase in anti-tTG in some coeliac subjects. CMP can produce this immune response if there were no gluten transgressions. This response has also been observed in non-IgE mediated CMP allergy patients with the susceptibility haplotype HLA DQ2/DQ8.
Los anticuerpos antitransglutaminasa (ATG) poseen alta especificidad para el diagnóstico de enfermedad celíaca (EC). Sin embargo, se han descrito anticuerpos ATG positivos en pacientes no celíacos.
ObjetivoValorar la presencia de anticuerpos ATG positivos no relacionados con la ingesta de gluten.
Pacientes y métodosRevisión retrospectiva de historias clínicas y seguimiento de pacientes con sospecha de EC y con un comportamiento serológico atípico, es decir, anticuerpos ATG positivos a pesar de una dieta sin gluten y disminución de anticuerpos ATG tomando gluten.
ResultadosSe incluyeron 9 casos. De ellos, 5 casos tenían afectación histológica Marsh 3 en la biopsia inicial y diagnóstico de EC (grupo A). Se retiró el gluten de la dieta y se retiraron las proteínas de leche de vaca (PLV) por la afectación nutricional. Al reintroducir las PLV aumentaron los ATG y al retirarlas se volvieron a normalizar. Los otros 4 pacientes presentaban una biopsia inicial normal (grupo B): en estos no se retiró el gluten, pero sí las PLV por sospecha de alergia no IgE mediada. Los síntomas desaparecieron y se normalizaron los ATG al retirar las PLV manteniendo dieta con gluten. Todos presentan el haplotipo de susceptibilidad para EC.
ConclusionesEn algunos celíacos, la reintroducción de PLV en la dieta tras un período de exclusión induce un aumento de los anticuerpos ATG IgA. Si se han descartado transgresiones con gluten, las PLV pueden causar esta respuesta inmune. Hemos observado también esta respuesta en pacientes con alergia no IgE, mediada por las PLV, portadores del haplotipo de susceptibilidad HLA DQ2/DQ8.
Anti-tissue transglutaminase (tTG) antibodies are highly specific for diagnosis of coeliac disease (CD).1,2 In fact, thanks to improvements in serological methods for the detection of these markers, and according to the new diagnostic criteria of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) of 2012, it may even be possible to omit performing an intestinal biopsy to diagnose CD under certain circumstances.3
However, despite the high specificity of anti-tTG antibodies for CD, there have been reports of the presence of these antibodies in patients without CD. The pathogenic mechanisms by which these antibodies are produced are not fully known, but they seem to differ between individuals with CD and individuals without. One of the possible mechanisms proposed is a “hapten-carrier model” by which gliadin would complex with transglutaminase in the intestinal mucosa, forming aggregates of macromolecules that would lead to synthesis of anti-tTG antibodies after being processed by B cells. These B cells with antibodies specific for tTG are likely to be present in all individuals, but are dependent on T cell help to start secreting immunoglobulin.4 Another hypothesis is that the production of transglutaminase is upregulated in areas of inflammation and could generate new antigenic epitopes by crosslinking or through deamidation of native proteins or proteins from exogenous sources, such as viruses, bacteria or food.5
There have been reports of transient anti-tTG antibody elevation in some autoimmune diseases and in patients with inflammatory bowel disease.6 Di Tola et al.7 found elevation of anti-tTG antibodies in more than 50% of the patients with inflammatory bowel disease in their study, all of who had negative results in the anti-endomysial antibody (EMA) test and low titres of anti-tTG antibodies compared to patients with CD; furthermore, they found an association between antibody titres and disease activity.
On the other hand, certain infectious agents can play a role in the production of anti-tTG antibodies. In a prospective study, Ferrara et al.8 found evidence of transient anti-tTG production in the context of infectious disease (Epstein–Barr virus and coxsackievirus infection) in patients with and without CD. The production of anti-tTG antibodies would reflect an immunologic process triggered by the overexpression of autoantigens or viral infection. None of the patients with a positive anti-tTG antibody test result had positive results for EMA, which supports the hypothesis that the immunologic mechanisms underlying anti-tTA antibody production are different in individuals with CD compared to those without.
Lastly, there is evidence in the literature that some dietary proteins, such as cow's milk protein (CMP), can cause gastrointestinal symptoms in some patients with CD. Bovine proteins can induce an inflammatory response in the mucosa of coeliac patients. This sensitivity to casein in patients with CD seems to be mediated by the innate immune response.9 These symptoms cannot be attributed to the presence of gluten in cow's milk, as gluten has not been detected in the milk of cows fed wheat.10 Instead, it seems that this response is due to the presence of epitopes shared by gliadins and bovine caseins that are recognised by immunoglobulin A (IgA) anti-gliadin antibodies (AGA).11,12 Casein has also been identified as a potential trigger in other autoimmune disorders.13
The aim of our study was to assess the presence of a positive anti-tTG antibody level not associated with gluten intake in a group of patients in followup for suspected CD.
Patients and methodsWe conducted a retrospective observational study by reviewing the cases of all patients managed in the outpatient clinics of our hospital between 2002 and 2010 in whom CD was initially suspected but whose serological patterns were atypical during the followup, that is, in whom changes in the levels of anti-tTG antibodies were not associated with gluten intake.
The inclusion criteria were having presented with elevation of anti-tTG or anti-gliadin IgA antibodies at the beginning of followup and having undergone an intestinal biopsy. We excluded patients in whom the antibody elevation had a demonstrated infectious aetiology and patients that did not undergo genetic testing. Group A included patients with characteristic histologic features in the intestinal biopsy in whom CD was diagnosed based on the EPSGHAN 1990 criteria. In this group, the serologic pattern was considered atypical in case of elevation of anti-tTG antibodies despite a gluten-free diet. Group B included patients with a normal intestinal biopsy who were placed on a CMP-free diet for diagnostic purposes due to suspicion of non-IgE-mediated CMP allergy. The diagnosis of CMP allergy was clinical and based on improvement of symptoms after exclusion of CMP and subsequent recurrence on a challenge test; the radioallergoabsorbent test (RAST) for cow's milk allergy was negative in all patients in whom it was requested. In this group, the serologic pattern was considered atypical in case the levels of anti-tTG antibodies decreased with the exclusion of CMP from the diet, despite not excluding gluten.
Anti-tTG IgA antibodies were measured using an automated fluorescence enzyme immunoassay method (EliA CeliKey, Phadia-Thermo Fisher; interpretation: negative <7U, positive >10U). We defined a strong positive result (++) as a titre greater than 10 times the upper limit of normal (>100U) and a weak positive result (+) as a positive result with a titre of less than 10 times the upper limit of normal (>7U and >100U). Anti-gliadin IgA antibodies were measured by means of an in-house ELISA method. The reference range, expressed in arbitrary units (AU), was less than 0.3AU in children and less than 0.6AU in adults.14
Anti-deamidated gliadin peptide antibodies were measured using an automated fluorescence enzyme immunoassay method (EliA AGP Phadia-Thermo Fisher; interpretation: negative <7U, positive >10U). The presence of EMA IgA antibodies was assessed by indirect immunofluorescence using sections of monkey oesophagus as substrate (Biosystems). Each test included a positive and a negative control. The serum was diluted 1:5 and incubated for 30min in a dark humid chamber, followed by incubation with FITC-conjugated anti-human IgA diluted to 1:20 for 30min. The sections were mounted in slides for performance of blinded fluorescence microscopy examination (Motic Trinocular BA400 fitted with a Motic FITC FLUO 3 filter cube).
Human leucocyte antigen (HLA) typing was performed by polymerase chase reaction for detection of DRB1, DQA1 or DQB1 alleles.
Histological findings were categorised according to the Marsh classification modified by Oberhuber.15 To detect subepithelial deposits of anti-tTG IgA, 5μm sections were prepared from intestinal biopsy specimens, frozen fresh, mounted on SuperFrost slides, and incubated (15min, humid chamber, room temperature) sequentially with: mouse monoclonal anti-transglutaminase 2 antibody (1:200 dilution), TRITC-conjugated rabbit anti-mouse immunoglobulin antibody (1:120 dilution) and FITC-conjugated anti-human IgA polyclonal rabbit antibody (1:40 dilution). This was followed by a blinded fluorescence microscopy examination with use of image overlay software (Motic Images Plus 2.0).
We made a retrospective review of the health records of these patients, retrieving data on the age at diagnosis, symptoms, anti-tTG antibody levels, HLA type, histologic features based on the Marsh-Oberhuber classification and changes in symptoms and levels of anti-tTG, AGA and EMA IgA antibodies based on the presence of gluten or CMP in the diet. We also retrieved data recorded during the followup to analyse changes in these parameters and attempt a definitive diagnosis.
The study protocol was approved by the Ethics Committee of the Hospital Universitari i Politècnic La Fe (Valencia).
We have expressed qualitative variables as absolute frequencies (n) and percentages, and summarised qualitative variables as median and interquartile range (IQR, 25th–75th percentile). We used the software SPSS® 21.0 to analyse the data.
ResultsOf the 629 patients assessed for suspected CD in the 2002–2010 period, 11 (1.7%) exhibited an atypical serologic pattern. We excluded 2 of these patients due to confirmation of an infectious aetiology (Giardia lamblia and Campylobacter jejuni). Intestinal infection was ruled out in the 9 remaining patients (1.4%). The median duration of followup was 10 years (IQR, 8–12.5).
We classified the patients into 2 groups. Group A included 5 patients with Marsh type 3 lesions in the initial biopsy that received a diagnosis of CD; a gluten-free diet was prescribed to these patients at the time of diagnosis. Group B included the remaining 4 patients, who had normal results in the initial biopsy and unremarkable histologic features, these patients were not asked to eliminate gluten from their diet.
A CMP-free diet was prescribed in both groups of patients for different reasons. In Group A, the reason for the diet was that the patients exhibited nutritional problems during the diagnostic evaluation of an intestinal malabsorption syndrome, and in group B the reason was suspicion that the cause of the gastrointestinal symptoms was a non-IgE-mediated allergy (Table 1).
Characteristics of the patients with an atypical serologic pattern and without an infectious aetiology.
| Group A | Group B | |
|---|---|---|
| n | 5 | 4 |
| Age at diagnosis (months) | ||
| Median | 15 | 20.5 |
| IQR | 14.5; 19.5 | 18.5; 29.5 |
| 1stintestinal biopsy | Marsh 3 | Marsh 0 |
| Treatment | GFD and CMPFD | CMPFD |
Group A: patients with abnormal histology in the first intestinal biopsy and a diagnosis of coeliac disease.
Group B: patients with normal findings in the intestinal biopsy and suspected non-IgE-mediated cow's milk protein allergy.
CMPFD, cow's milk protein-free diet; GFD, gluten-free diet; IQR, interquartile range.
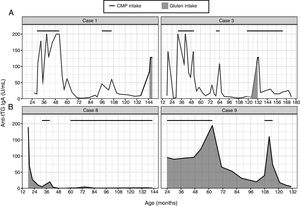
In group A, once the results of the intestinal biopsy became available and CD was diagnosed, gluten was excluded from the diet. The patients exhibited a decrease in anti-tTG antibody levels. Subsequently, once the nutritional status of these patients had improved, CMP was reintroduced in the diet, and there was an associated increase in the levels of anti-tTG antibodies that had previously become negative, while the levels of EMA antibodies were negative (Fig. 1A). Of these patients, one had diarrhoea and the rest were asymptomatic. The results were confirmed in all of these patients on retesting of different specimens after ruling out non-adherence to the gluten-free diet (dietary interview and negative AGA antibody levels). After excluding CMP from the diet again, serologic markers returned to normal. After a second challenge with cow's milk, patient 3 continued to have negative anti-tTG results, while patients 1, 2 and 4 had positive results again. Patient 5 is still undergoing evaluation. In 3 out of the 5 patients in this group, CD was confirmed with a gluten challenge (patients 1, 2 and 3). Of the 2 remaining patients, 1 refused to undergo the gluten challenge (patient 4), and patient 5 is undergoing the gluten challenge at the time of this writing. In patients 1 and 2, sensitisation to bovine proteins persisted at 12 and 8 years of followup, respectively (Table 2). Patient 4 consumes CMP and her anti-tTG antibody levels continue to be negative, so her sensitivity to CMP resolved within 5 years.
Group A: patients with abnormal histology in the initial intestinal biopsy.
| Patient | Age (months) | Presentation | Serologic markers | HLA | 1st intestinal biopsy | Gluten challenge | Diagnosis | Duration of followup (years) |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | MS | AGA+ | DQ2 | Marsh 3B | Positive; Marsh 3A | CD and sensitisation to CMP | 14 |
| 2 | 15 | MS | AGA+ atTG++ EMA+ | DQ2/DQ2 | Marsh 3B | Positive | CD and sensitisation to CMP | 10 |
| 3 | 19 | MS | AGA+ atTG++ EMA+ | DQ2 | Marsh 3B | Symptoms after challenge | CD and transient sensitisation to CMP | 13 |
| 4 | 14 | Weight loss | AGA+ atTG++ EMA+ | DQ2 | Marsh 3C | Refused | CD and transient sensitisation to CMP | 8 |
| 5 | 15 | MS | atTG+ | DQ2 | Marsh 3C | Undergoing | Pending | 8 |
AGA, anti-gliadin IgA antibodies; atTG, anti-transglutaminase IgA antibodies; CD, coeliac disease; CMP, cow's milk protein; EMA, anti-endomysial IgA antibodies; HLA: human leucocyte antigen; MS, malabsorption syndrome.
In group B, removal of CMP from the diet was followed by resolution of symptoms, with anti-tTG and EMA antibody values becoming negative (Fig. 1B). In 2 of these patients (6 and 7), serologic markers remained negative and symptoms did not recur on reintroduction of CMP in the diet. On the other hand, the other 2 patients (8 and 9) experienced a recurrence of symptoms and elevation of anti-tTG antibodies on reintroduction of CMP. Patient 8 underwent a second CMP challenge a year later, remaining asymptomatic and with negative anti-tTG antibody levels. Patient 9 underwent a second CMP challenge following the same procedure, after which testing showed iron deficiency and positive anti-tTG and EMA values. This led to performance of an intestinal biopsy that revealed Marsh 3 B lesions and subepithelial deposits of anti-tTG IgA in the intestinal mucosa. Despite these findings, CMP was removed from the diet again, with normalisation of anti-tTG and EMA values. The patient is currently scheduled for an intestinal biopsy to rule out latent CD (Table 3).
Group B: patients with a normal intestinal biopsy and suspected non-IgE-mediated cow's milk protein allergy.
| Patient | Age (months) | Presentation | Serologic markers | HLA | 1st intestinal biopsy | 2nd intestinal biopsy | Diagnosis | Duration of followup (years) |
|---|---|---|---|---|---|---|---|---|
| 6 | 21 | Weight loss | atTG+ AGA− | DQ2/DQ2 | No specimen | Transient sensitisation to CMP | 9 | |
| 7 | 32 | Chronic diarrhoea | atTG+ AGA+ EMA− | DQ2 | Normal | Transient sensitisation to CMP | 6 | |
| 8 | 18 | MS | atTG++ AGA+ EMA+ | DQ2 | Normal | Transient sensitisation to CMP | 12 | |
| 9 | 20 | MS | atTG++ AGA− EMA− | DQ2 | Normal | Marsh 3 B; IgA deposits | Enteropathy susceptible to CMP vs potential CD | 12 |
AGA, anti-gliadin IgA antibodies; atTG, anti-transglutaminase IgA antibodies; CD, coeliac disease; CMP, cow's milk protein; EMA, anti-endomysial IgA antibodies; HLA: human leucocyte antigen; MS, malabsorption syndrome.
In the sample under study, we found that anti-tTG antibody elevation occurred as part of an immune response to dietary proteins other than gluten both in coeliac patients (group A) and patients without CD (group B).
In the patients with CD, this elevation of anti-tTG antibodies coincided with the reintroduction of CMP in the diet. Having ruled out gluten intake as the cause for this elevation by asking patients about their adherence to the gluten-free diet in successive visits, the elevation persisted in association with negative levels of AGA antibodies. At present, recently introduced methods for detection of gluten peptides in stool would allow us to confirm dietary compliance.16
When patients returned to the bovine milk-free diet, the anti-tTG antibody levels became negative again. This finding supports our hypothesis that CMP can trigger this immune response, as has been previously described in the literature. Cabrera-Chávez et al. presented a hypothesis of how certain epitopes in CMP could trigger symptoms in patients with CD. The high homology of some peptides in the α and ß chains of bovine caseins with gluten peptides could explain this IgA immunoreactivity.11,12
This response was transient in 2 of the 5 patients with CD, patients 3 and 4. However, it has persisted to date in another 2, patients 1 and 2, currently aged 12 and 8 years, respectively, who have been on a gluten-free diet from the time of diagnosis. In untreated patients with CD, this response could be explained by the increased passage of macromolecules through the intestinal barrier. However, in patients with CD under a gluten-free diet with a healthy intestinal mucosa, it is not clear whether this could be due to the persistence of increased intestinal permeability or to other proinflammatory factors.7
All patients with CD in whom this response was observed had been following a CMP-free diet, which could have been the cause of the subsequent reactivity. Furthermore, they were all aged 2 years or less, an age band in which there is increased reactivity to dietary antigens.17,18 Thus, we should consider whether a CMP-free diet should be avoided in patients in whom CD is strongly suspected.
The atypical serologic pattern in patients in group B could be due to different mechanisms, as there was no evidence that they had CD, although they all had HLA types associated with a higher risk of CD. As described in the literature, some infectious agents can also trigger this immune response, resulting in elevation of anti-tTG antibodies in patients without CD.8 However, we did not find any reference in the literature of dietary bovine proteins as a potential trigger of anti-tTG antibody elevation in non-coeliac patients, as was the case of the patients in group B of our study. All of these patients were carriers of HLA-DQ2 or DQ8. Could casein have a similar role to that of gliadin in patients with CD, only not damaging the intestinal mucosa? As we noted above, an immune response to casein may be involved in the pathogenesis of some autoimmune disorders, such as Behçet disease, through modulation of T cells and macrophages.13
In 80% of our sample, this response was transient. However, the response persisted in patient 9, with eventual damage to the intestinal mucosa including evidence of IgA deposits, so that latent CD cannot be ruled out.
In addition to elevation of anti-tTG antibody levels, patient 8 had positive AGA and EMA values that subsequently resolved, with no histologic evidence of CD and permanent serologic normalisation with a diet from which gluten was not excluded. Patient 7 also had transient positive AGA values. This could be explained by the increased intestinal permeability exhibited by children with inflammation secondary to infection or food allergies. In these patients, the process that triggered the development of a non-IgE-mediated allergy to CMP was probably an infectious gastroenteritis, as both patients had previously been consuming CMP without problems and the intolerance appeared at 32 and 18 months. The infection itself may have also been the cause of the elevation of these markers, as has been described previously.19
Adherence to a strict CMP-free diet is challenging in today's society, possibly more so than adherence to a gluten-free diet. The labelling of food products is confusing. Cow's milk protein may be present in all types of foods in the form of various additives that have obscure names. Thus, it would be useful to know of a marker that could help determine whether this immune response could be due to CMP in coeliac patients in whom non-adherence to the gluten-free diet has been ruled out, before adding more restrictions to the diet. On the other hand, since this seems to be an IgA-mediated response, one would expect these patients to have higher levels of casein-specific IgA. The published evidence on this subject shows that IgG and IgA antibodies to bovine proteins are more elevated in patients with untreated CD compared to patients with CD following a gluten-free diet and with a healed mucosa, who exhibit lower antibody levels,17 although further research is needed to confirm this association.
The sample in our study was small, as we only included patients that had undergone an intestinal biopsy managed at a single hospital. However, the long duration of followup allowed us to detect this atypical pattern.
In our experience, in some patients with CD, reintroduction of CMP in the diet after a period of exclusion induced an increase in anti-tTG IgA antibody levels, which was asymptomatic in most cases. We believe that once non-adherence to the gluten-free diet has been ruled out, CMP should be considered as a potential trigger of this immune response. We also found this response in patients with non-IgE-mediated allergy to CMP that were HLA-DQ2/DQ8 carriers.
In conclusion, anti-tTG antibodies may be found in patients with diseases other than CD, and it is important that clinicians consider the potential role of food antigens, especially CMP, in these cases.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the Biostatistics Unit of the Instituto de Investigación Sanitaria La Fe.
Please cite this article as: Garcia-Peris M, Donat Aliaga E, Roca Llorens M, Masip Simó E, Polo Miquel B, Ribes Koninckx C. Anticuerpos antitransglutaminasa no relacionados con la ingesta de gluten. An Pediatr (Barc). 2018;89:279–285.
Previous presentations: This study was presented as an oral communication with the title “Anticuerpos antitransglutaminasa elevados. ¿Enfermedad celíaca o sensibilización a las proteínas de leche de vaca?” at the XX Congress of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP), 2013, Spain; as an oral communication with the title “Anticuerpos antitransglutaminasa elevados no relacionados con la ingesta de gluten” at the IV National Congress of the Sociedad Española de Enfermedad Celíaca (SEEC), November 2014, Valencia, Spain; and as a poster titled “Are antitransglutaminase antibodies related always to gluten ingestion?” at the International Celiac Disease Symposium (ICDS), June 21–14, 2015, Prague, Czech Republic.