Health care-associated infections are common in neonatology, but there is no consensus on their definitions. This makes it difficult to compare their incidence or assess the effectiveness of prevention bundles. This is why we think it is very important to achieve a consensus on the definitions and diagnostic criteria for one of the most frequent causes of morbidity in hospitalised neonates. This document aims to standardise the definitions for the most frequent health care-associated infections, such as catheter-associated bloodstream infection, ventilator-associated pneumonia and surgical wound infection, as well as the approach to their diagnosis and treatment.
Las infecciones relacionadas con la asistencia sanitaria son frecuentes en neonatología pero no existe un consenso en sus definiciones. Esto dificulta la comparación de incidencias entre distintas unidades o la valoración de la eficacia de los paquetes de prevención. Es por ello que consideramos muy importante lograr un consenso en las definiciones y diagnóstico de una de las morbilidades más frecuentes de los neonatos hospitalizados. El presente documento pretende unificar estas definiciones en relación a las infecciones más frecuentes como son la bacteriemia relacionada con el catéter, la neumonía asociada a la ventilación mecánica y la infección de la herida quirúrgica, así como su abordaje diagnóstico-terapéutico.
Nosocomial infections, also known as health care-associated infections (HCAIs), are caused by microorganisms present in the hospital setting transmitted by direct contact or through fomites. The most frequent ones are those associated with medical devices, such as catheter-related bloodstream infections (CRBSIs) and ventilator-associated pneumonia (VAP), to which very low birth weight (VLBW) infants are most susceptible.1 The aim of the present document is to standardise the definitions and the approach to the diagnosis and management of these diseases.
Catheter-related bloodstream infectionDefinitions. EpidemiologyNeonatal sepsis is defined as the development of systemic inflammatory response syndrome (SIRS) (Table 1) secondary to the presence of an infectious agent, usually in the blood. Although there is no international consensus definition,2 early-onset sepsis and late-onset sepsis are usually defined based on whether the symptoms develop before or after 72 h post birth.3 However, it seems less arbitrary to base the classification on the mechanism of transmission, thus referring to vertical sepsis (mother-infant transmission) and nosocomial sepsis (acquired in hospital), independently of the timing of onset.4
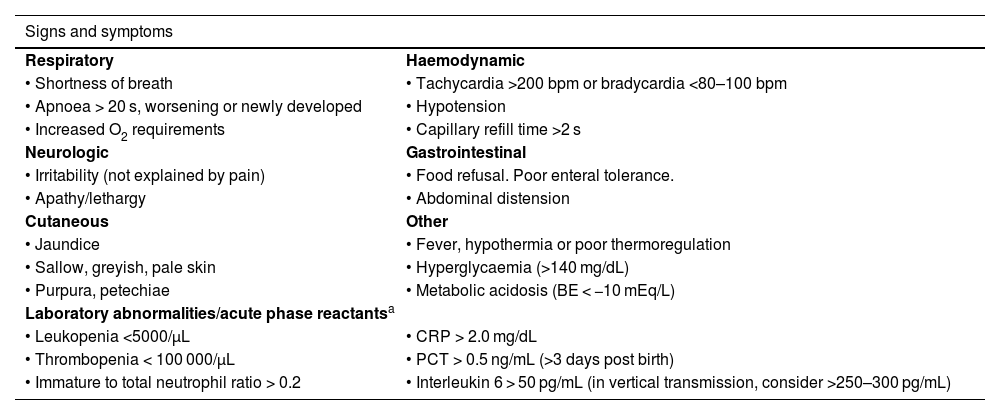
Clinical criteria for suspicion of sepsis.2
| Signs and symptoms | |
|---|---|
| Respiratory | Haemodynamic |
| • Shortness of breath | • Tachycardia >200 bpm or bradycardia <80–100 bpm |
| • Apnoea > 20 s, worsening or newly developed | • Hypotension |
| • Increased O2 requirements | • Capillary refill time >2 s |
| Neurologic | Gastrointestinal |
| • Irritability (not explained by pain) | • Food refusal. Poor enteral tolerance. |
| • Apathy/lethargy | • Abdominal distension |
| Cutaneous | Other |
| • Jaundice | • Fever, hypothermia or poor thermoregulation |
| • Sallow, greyish, pale skin | • Hyperglycaemia (>140 mg/dL) |
| • Purpura, petechiae | • Metabolic acidosis (BE < −10 mEq/L) |
| Laboratory abnormalities/acute phase reactantsa | |
| • Leukopenia <5000/μL | • CRP > 2.0 mg/dL |
| • Thrombopenia < 100 000/μL | • PCT > 0.5 ng/mL (>3 days post birth) |
| • Immature to total neutrophil ratio > 0.2 | • Interleukin 6 > 50 pg/mL (in vertical transmission, consider >250–300 pg/mL) |
BE, base excess; bpm, beats per minute; CRP, C-reactive protein; PCT, procalcitonin.
According to data from the Castrillo Group nationwide network in Spain, the incidence of nosocomial sepsis is less than 2% in neonates with a birth weight (BW) greater than 1500 g compared to 20%–30% in neonates with a BW of 1500 g or less or even 40%–50% in neonates with a BW below 1000 g,4 figures that are similar to those reported by other studies in different countries.5
The incidence of CRBSI in our nationwide network is of 5.6 episodes per 1000 catheter days for peripherally inserted central catheters and 7.3 episodes per 1000 catheter days for umbilical catheters.4 These figures may vary between case series depending on the definitions each of them apply,6 to the point that some report near-zero rates.7
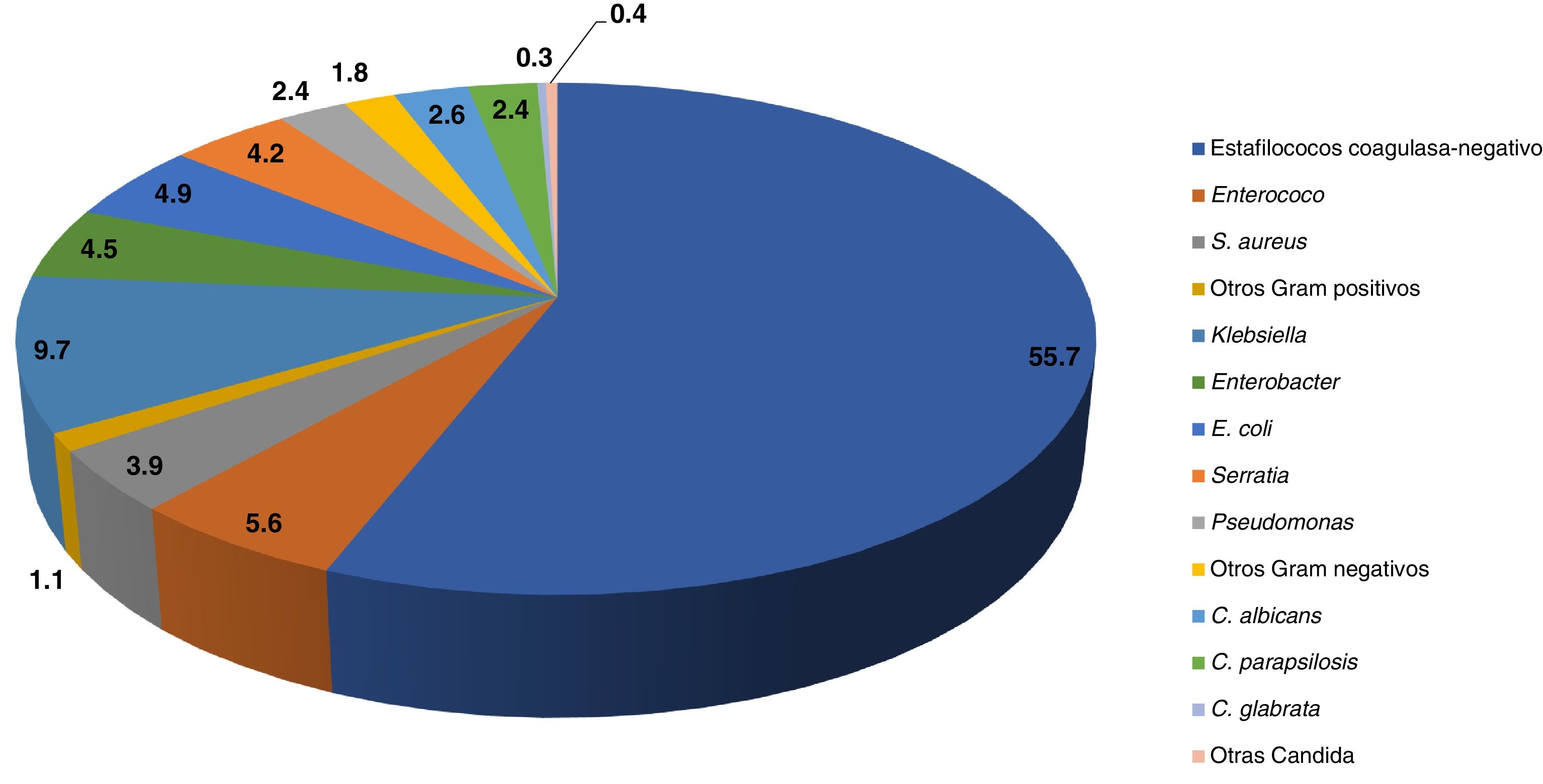
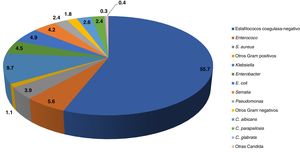
The most prevalent aetiological agents in nosocomial sepsis nosocomial are gram-positive bacteria (66%) (especially coagulase-negative staphylococci [CoNS]), followed by gram-negative bacteria (28%) (especially Enterobacteriaceae) and Candida species (6%) (Fig. 1).4
Diagnostic criteriaNosocomial sepsis: systemic inflammatory response syndrome in a hospitalised neonate with isolation in blood culture of a microorganism found in the hospital setting, and absence of infection, either active or latent, at the time of hospital admission. It usually develops from 3 days after admission. Although blood culture continues to be the gold standard for diagnosis of sepsis, at present there are molecular methods based on the detection of bacterial DNA or RNA with a high sensitivity and specificity that offer advantages in terms of the turnaround time and the capacity to identify the organism,8 although they may yield false negative (bacteria not included in the panel) and false positive (contaminated sample) results.9
- •
In the case of CoNS isolation: the following is required to rule out contamination: separate collection of 2 peripheral blood samples, with positive results in both (preferable) or positive results in both culture of a peripheral blood sample and blood drawn from the catheter. If obtaining 2 separate blood samples is complicated, it is possible to obtain two samples from a single venepuncture, changing the phlebotomy supplies and seeding 2 different culture bottles, and ruling out contamination if the same CoNS is isolated from both.4 Other protocols only require meeting various criteria for SRIS and ruling out contamination of the blood culture.10 On the other hand, a prolonged time to blood culture positivity (>16 h) and an inadequate sample volume (<0.5−1 mL) make contamination more likely.11
Clinical nosocomial sepsis: systemic inflammatory response syndrome in a hospitalised neonate with negative blood culture results in whom empiric antibiotherapy is maintained for at least 5 days because infection seems the most plausible explanation of the clinical presentation.12
Catheter-associated bloodstream infection: detection of the same microorganism in blood cultures from peripheral vein and catheter tip samples (if the catheter is withdrawn) or from samples obtained from the hub and/or skin around the insertion site (if the catheter is not withdrawn).13 If the catheter is not withdrawn, 2 blood samples can be obtained, one from a peripheral vein and the other from the central venous catheter, within the same hour and extracting the same volume of blood. A colony count 3–4 times greater in the blood culture of the catheter sample compared to the peripheral vein sample or positivity at least 2 times earlier in the blood culture of the sample obtained from the catheter compared to the peripheral vein sample supports the diagnosis of CRBSI. In the presence of clinical manifestations compatible with sepsis, it is possible to contemplate catheter-associated sepsis (CAS).
Catheter-associated bloodstream infection/sepsis: nosocomial bloodstream infection/sepsis in a patient with an indwelling intravenous catheter or in whom the catheter has been removed in the past 48 h.13
ManagementWhenever there is clinical suspicion of sepsis, in addition to the collection of a sample for blood culture before initiation of antibiotherapy, performance of lumbar puncture is recommended for biochemical and microbiological testing of cerebrospinal fluid. When it comes to empiric treatment, the combination of 2 antibiotics is recommended: one covering gram-positive bacteria and another covering gram-negative bacteria. Each unit must be aware of the antimicrobial susceptibility profile of the microorganisms colonising the environment and select the regimen that provides the best coverage. Based on the causative agents involved in nosocomial sepsis reported to the Castrillo Group, the best combination would consist of a glycopeptide (vancomycin or teicoplanin) and an aminoglycoside (amikacin or gentamicin). An aminoglycoside is preferable to the use of third-generation cephalosporins on account of the risk of bacterial drug resistance and candidiasis. In units with “zero tolerance” programmes and a low incidence of nosocomial sepsis, the use of cloxacillin instead of a glycopeptide could be considered for empiric treatment to prevent the development of vancomycin resistance and an increase in the incidence of sepsis caused by gram-negative bacteria.14 Once the susceptibility profile has been established, the definitive treatment will be adjusted to the narrowest possible spectrum based on the antimicrobial susceptibility testing results. If the blood culture is negative, prompt discontinuation of antibiotherapy should be considered.
In the case of candidaemia, the first-line treatment is amphotericin B deoxycholate or a less toxic lipid-based formulation (amphotericin B liposomal or lipid complex). Other possible options are micafungin (in the case of a poor response or significant toxicity) or fluconazole, although the latter is not an option if the patient has received the drug for prophylaxis.15
In patients with CAS, routine removal of the catheter is not recommended, especially in patients in whom it would be complicated to place a new catheter, except in the following cases13:
- -
Patients with septic shock once it has resolved.
- -
Catheter complications: local, thrombophlebitis, endocarditis, metastatic infection of any organ (bone, skin, liver…).
- -
Isolation of Candida, Staphylococcus aureus, Bacillus subtilis or gram-negative bacteria, especially if they are multidrug-resistant. For other microorganisms, the need for removal will be assessed on a case-by-case basis.
- -
Persistent bacteraemia/fungaemia, without clinical improvement or worsening, after 48–72 h of appropriate treatment.
In cases in which the catheter cannot be withdrawn, it is advisable to perform a follow-up culture every 24–48 h, and if it continues to be positive, the catheter should be removed. In addition, in patients with infection by Candida spp., a new catheter should not be inserted until several doses of antifungal have been administered.13,15
PreventionPreventive efforts must focus on avoiding colonization of the infant by pathogens present in the hospital environment. When it comes to the prevention of CRBSI in particular, different care bundles have been found to achieve significant reductions in their incidence.16,17Table 2 presents the most important preventive measures.
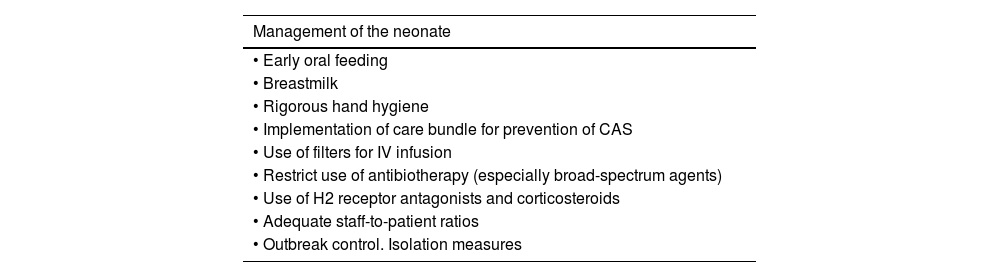
Prevention measures for nosocomial sepsis.
| Management of the neonate |
|---|
| • Early oral feeding |
| • Breastmilk |
| • Rigorous hand hygiene |
| • Implementation of care bundle for prevention of CAS |
| • Use of filters for IV infusion |
| • Restrict use of antibiotherapy (especially broad-spectrum agents) |
| • Use of H2 receptor antagonists and corticosteroids |
| • Adequate staff-to-patient ratios |
| • Outbreak control. Isolation measures |
| Pharmacological management |
|---|
| • Probiotics |
| • Fluconazole in high-risk VLBW newborns |
| • Lactoferrin (currently in research), alone or combined with probiotics |
| Care bundle for prevention of CABSI |
|---|
| • Previous training of any staff handling CVCs |
| • Hand hygiene any time catheters, connectors or bandages are handled |
| • Use of aseptic technique for CVC placement and handling (sterile cap, mask, robe and gloves) |
| • Skin disinfection with 2% chlorhexidine |
| • Use of semipermeable bandages allowing visualization of site of insertion, changing bandages whenever they appear soiled or at least every 7 days |
| • Change parenteral nutrition tubes, extension lines and filters every 24 h |
| • Disinfect connectors with alcoholic chlorhexidine before and after use |
CABSI, catheter-associated blood stream infection; CAS, catheter-associated sepsis; CVC, central venous catheter; IV, intravenous; VLBW, very low birth weight.
Other pharmacological measures that have proven efficacious are oral supplementation with probiotics, whose immunomodulating and anti-infective properties make them effective for prevention of necrotising enterocolitis18 and the use of fluconazole in VLBW infants for prevention of invasive fungal infections (achieving a reduction in the incidence of fungal infections of up to 90%).19 The use of lactoferrin, alone or combined with probiotics, is currently being investigated, as it may be able to reduce the incidence of nosocomial sepsis and enterocolitis by promoting the growth of beneficial enteral bacteria and intestinal maturation in addition to its fungicidal activity.20
Ventilator-associated pneumoniaDefinitionVentilator-associated pneumonia (VAP) refers to pneumonia occurring in patients who have been on mechanical ventilation (MV) for at least 48 consecutive hours. It is the second most frequent nosocomial infection, amounting to 20%–30% of HCAIs. It should be assessed in terms of incidence density (VAP episodes/1000 ventilator days) and the device utilization ratio (ventilator days/inpatient days).21
The incidence in newborns is greater compared to other age groups, ranging between 2.7 and 37.2 episodes/1000 ventilator days depending on the unit and the applied definition criteria. The most recent International Nosocomial Infection Control Consortium report calculated an incidence of 9.02 episodes/1000 ventilator days.22 In some cases, the aetiology may be polymicrobial, with S. aureus and Pseudomonas aeruginosa being most frequently involved.23
Ventilator-associated pneumonia usually develops after 20–30 days of intubation and is associated with an increased length of stay and, in some studies, an increase in mortality.24
Diagnostic criteriaThe diagnosis of VAP requires fulfilment of all the criteria established by the Centers for Disease Control and Prevention (CDC) for children aged less than 1 year, and specific criteria have not been established for neonates (Table 3).21
Diagnostic criteria for ventilator-associated pneumonia in children aged less than 1 year, Centers for Disease Control and Prevention (CDC).21
| Radiological diagnosis | Chest radiograph (or 2 or more in patients with underlying disease) with one of the following features: |
| • New or progressive persistent infiltration | |
| • Consolidation | |
| • Cavitation | |
| • Pneumatocoeles | |
| Clinical diagnosis | Worsening gas exchange (desaturations, increased oxygen requirements or increased ventilator demand) and 3 of the following signs: |
| • Temperature instability with no other cause | |
| • Leukopenia (<4000 WBC/mm³) or leucocytosis (>15 000 WBC/mm³) and left shift (>10% band forms) | |
| • New onset of purulent sputum (≥25 neutrophils and ≤10 squamous epithelial cells/low power field), change in character of sputum, increased respiratory secretions or increased suctioning requirements | |
| • Apnoea, tachypnoea, nasal flaring with chest wall retraction or grunting | |
| • Wheezing, rales or rhonchi | |
| • Cough | |
| • Bradycardia (<100 bpm) or tachycardia (>170 bpm) | |
| Microbiological diagnosis | At least one of the following: |
| • Positive blood culture with no other source of infection | |
| • Positive pleural fluid culture | |
| • Positive quantitative culture of a minimally contaminated/bronchoscopically obtained LRT specimen (BAL [≥104 cfu/mL], protected specimen brushing [≥103 cfu/mL]) or, when not available, endotracheal aspirate sample (≥106 cfu/mL) | |
| • ≥ 5% BAL-obtained cells contain intracellular bacteria on direct microscopic exam (Gram stain) | |
| • Histopathologic exam showing at least one of the following evidences of pneumonia: | |
| ○ Abscess formation or foci of consolidation with intense PMN accumulation in bronchioles and alveoli | |
| ○ Positive quantitative culture of lung tissue (≥104 cfu/g of tissue) or evidence of lung parenchyma invasion by fungal hyphae or pseudohyphae |
BAL, bronchoalveolar lavage; cfu, colony-forming units; LRT, lower respiratory tract; PMN, polymorphonuclear leukocyte; WBC, white blood cell.
The diagnosis of VAP in newborns is particularly challenging because underlying lung disease is common, so the imaging criterion must be based on the presence of abnormal features in at least 2 successive imaging tests. The microbiological criterion is essential to confirm the diagnosis of VAP. Cases that meet all the criteria with the exception of microbiological confirmation will be defined as clinical VAP. Still, some authors warn of the risk of overdiagnosis if the microbiological isolation criterion is forfeited.25 In addition, while some studies have used culture or the presence of purulent secretions in endotracheal aspirate samples for diagnosis of VAP, other authors insist in the need of obtaining minimally contaminated lower respiratory tract specimens to avoid the diagnosis of infection in cases in which the pathogen is only present in the upper respiratory tract.26
ManagementSpecific guidelines for the paediatric or neonatal populations are not available, except for local recommendations, and both the United States27 and European28 clinical practice guidelines were developed for the adult population. Both recommend collection of respiratory samples for culture and selection of broad-spectrum empiric treatment regimens informed by the local distribution of pathogens associated with VAP, switching to narrow-spectrum targeted therapy once the causative agent is identified. Combining penicillin, glycopeptide or linezolid with an aminoglycoside or piperacillin-tazobactam could be effective for initial treatment, to be switched by the most appropriate targeted agent based on the identified pathogen. If there is no response, second-line treatment could consist of a cephalosporin with anti-pseudomonal activity, and cotrimoxazole could be contemplated in the case of cephalosporin-resistant Stenotrophomonas maltophilia.29
The optimal duration of treatment has not been clearly established, although both guidelines recommend a 7-day course for uncomplicated pneumonia. A study aimed at reducing antibiotic use used a short 5-day course for uncomplicated cases of pneumonia with negative cultures and did not find evidence of reinfection or an increase in mortality.30
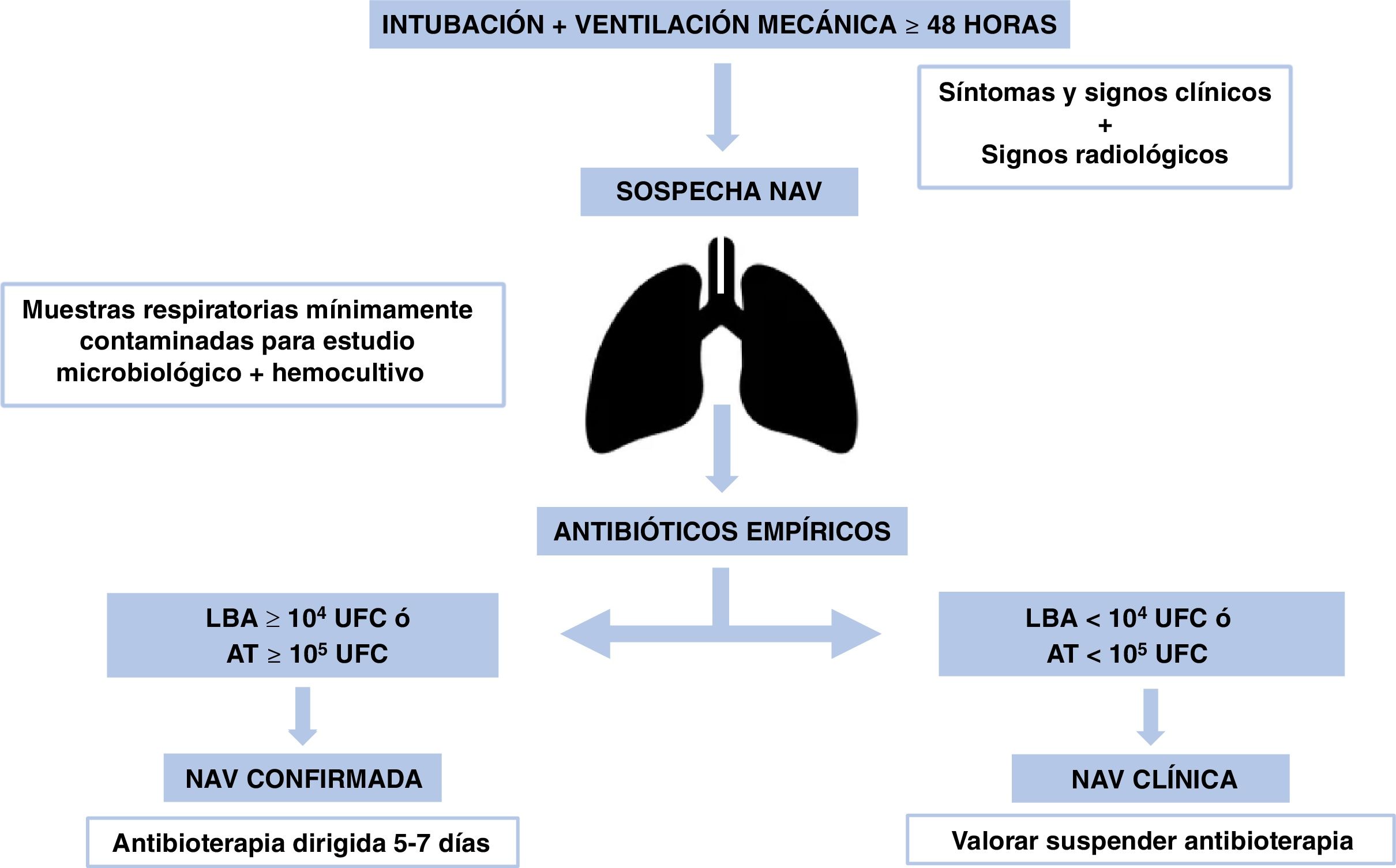
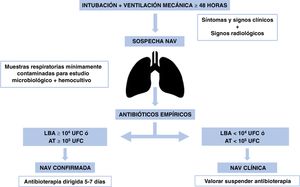
In patients with negative cultures and no clinical worsening, empiric antibiotherapy should be discontinued,31 as most suspected VAP cases are not confirmed microbiologically.32Fig. 2 presents the algorithm for the management of VAP.
PreventionBelow is a series of measures that have been found to significantly reduce the incidence of VAP in neonates33,34:
- 1.
Sterile technique during intubation and aspiration, avoiding routine use of saline solution.
- 2.
Suction of oropharyngeal secretions before endotracheal tube handling. Use of a double suction system for independent suction of oral cavity and of airway secretions.
- 3.
Avoiding reintubation. Daily assessment of need to continue mechanical ventilation.
- 4.
Positioning measures, trying the lateral position. In patients with gastro-oesophageal reflux, elevation of the head of the bed by 15° to 30°.
- 5.
Cleaning of gums, tongue, and lips every 3 or 4 h and before endotracheal intubation or gastric tube insertion. Some evidence supports the use of bicarbonate solution or colostrum instead of double-distilled water, as they make the environment more alkaline and increase the concentrations of lactoferrin and IgA35; while other studies, while having observed these effects, have not found a reduction in the colonization by VAP-causing pathogens in extremely preterm infants.36
- 6.
Change of breathing circuit at the intervals recommended by the manufacturer or when visibly soiled. Cleaning of endotracheal tube connections (bag-valve mask) with alcohol and clearance of condensation from the tubing without disconnecting from ventilator, use of heating.33
Surgical site infection (SSI) is defined as infection of the surgical incision space, which may involve the skin, subcutaneous tissue (localised erythema, swelling, pain or purulent secretion at the site of surgery or near it, with or without fever) or deeper tissues in the area in the 30 days following the procedure (in the case of prostheses or foreign bodies, it may develop within up to 90 days).37
The prevention of SSIs is based on adequate surgical technique, sterile and aseptic technique and surgical antibiotic prophylaxis (SAP).
Surgical antibiotic prophylaxis consists in the administration of antibiotherapy prior to the surgical intervention to reduce the risk of SSI and is indicated depending on the type of surgery to be performed and the risk factors present in each neonate.
Classification of the type of surgery for the selection of antibiotic prophylaxis or treatment based on the risk of SSI is showed in Table 4.
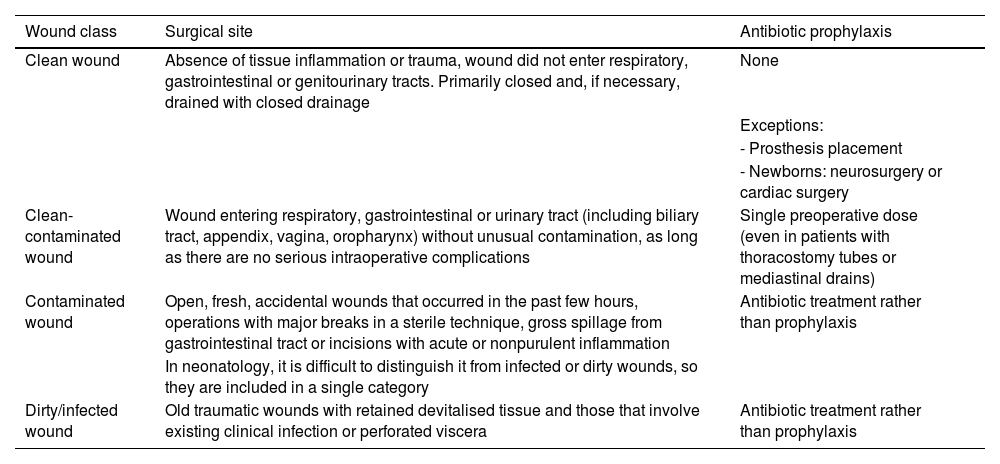
Surgical wound classification for the selection of antibiotic prophylaxis or treatment based on the risk of surgical site infection.37
| Wound class | Surgical site | Antibiotic prophylaxis |
|---|---|---|
| Clean wound | Absence of tissue inflammation or trauma, wound did not enter respiratory, gastrointestinal or genitourinary tracts. Primarily closed and, if necessary, drained with closed drainage | None |
| Exceptions: | ||
| - Prosthesis placement | ||
| - Newborns: neurosurgery or cardiac surgery | ||
| Clean-contaminated wound | Wound entering respiratory, gastrointestinal or urinary tract (including biliary tract, appendix, vagina, oropharynx) without unusual contamination, as long as there are no serious intraoperative complications | Single preoperative dose (even in patients with thoracostomy tubes or mediastinal drains) |
| Contaminated wound | Open, fresh, accidental wounds that occurred in the past few hours, operations with major breaks in a sterile technique, gross spillage from gastrointestinal tract or incisions with acute or nonpurulent inflammation | Antibiotic treatment rather than prophylaxis |
| In neonatology, it is difficult to distinguish it from infected or dirty wounds, so they are included in a single category | ||
| Dirty/infected wound | Old traumatic wounds with retained devitalised tissue and those that involve existing clinical infection or perforated viscera | Antibiotic treatment rather than prophylaxis |
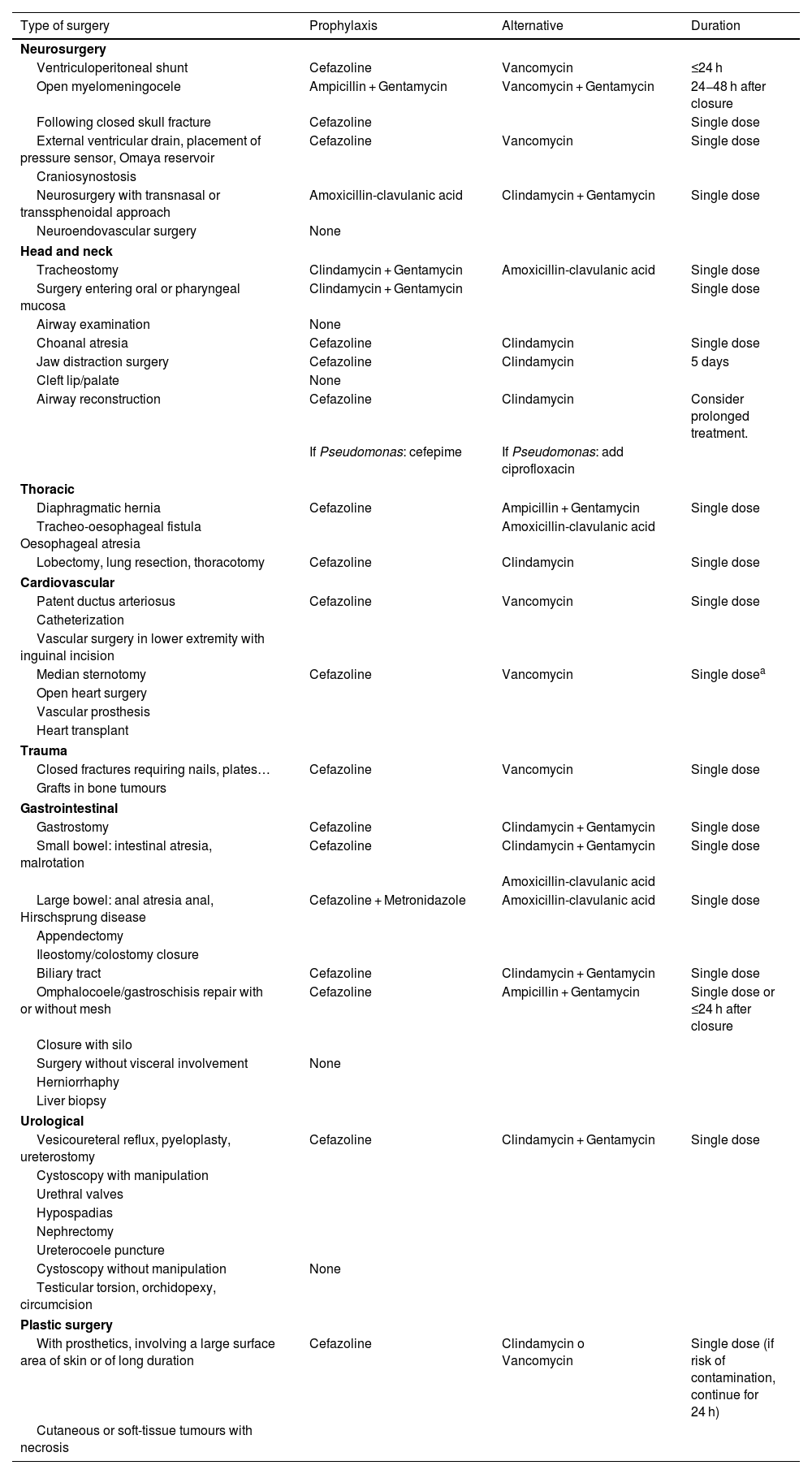
Adequate selection of SAP includes the type of antibiotic, the route of administration and the number of doses (see Table 5).38,39
Recommended surgical prophylaxis based on surgical site and type of surgery.38–42
| Type of surgery | Prophylaxis | Alternative | Duration |
|---|---|---|---|
| Neurosurgery | |||
| Ventriculoperitoneal shunt | Cefazoline | Vancomycin | ≤24 h |
| Open myelomeningocele | Ampicillin + Gentamycin | Vancomycin + Gentamycin | 24−48 h after closure |
| Following closed skull fracture | Cefazoline | Single dose | |
| External ventricular drain, placement of pressure sensor, Omaya reservoir | Cefazoline | Vancomycin | Single dose |
| Craniosynostosis | |||
| Neurosurgery with transnasal or transsphenoidal approach | Amoxicillin-clavulanic acid | Clindamycin + Gentamycin | Single dose |
| Neuroendovascular surgery | None | ||
| Head and neck | |||
| Tracheostomy | Clindamycin + Gentamycin | Amoxicillin-clavulanic acid | Single dose |
| Surgery entering oral or pharyngeal mucosa | Clindamycin + Gentamycin | Single dose | |
| Airway examination | None | ||
| Choanal atresia | Cefazoline | Clindamycin | Single dose |
| Jaw distraction surgery | Cefazoline | Clindamycin | 5 days |
| Cleft lip/palate | None | ||
| Airway reconstruction | Cefazoline | Clindamycin | Consider prolonged treatment. |
| If Pseudomonas: cefepime | If Pseudomonas: add ciprofloxacin | ||
| Thoracic | |||
| Diaphragmatic hernia | Cefazoline | Ampicillin + Gentamycin | Single dose |
| Tracheo-oesophageal fistula Oesophageal atresia | Amoxicillin-clavulanic acid | ||
| Lobectomy, lung resection, thoracotomy | Cefazoline | Clindamycin | Single dose |
| Cardiovascular | |||
| Patent ductus arteriosus | Cefazoline | Vancomycin | Single dose |
| Catheterization | |||
| Vascular surgery in lower extremity with inguinal incision | |||
| Median sternotomy | Cefazoline | Vancomycin | Single dosea |
| Open heart surgery | |||
| Vascular prosthesis | |||
| Heart transplant | |||
| Trauma | |||
| Closed fractures requiring nails, plates… | Cefazoline | Vancomycin | Single dose |
| Grafts in bone tumours | |||
| Gastrointestinal | |||
| Gastrostomy | Cefazoline | Clindamycin + Gentamycin | Single dose |
| Small bowel: intestinal atresia, malrotation | Cefazoline | Clindamycin + Gentamycin | Single dose |
| Amoxicillin-clavulanic acid | |||
| Large bowel: anal atresia anal, Hirschsprung disease | Cefazoline + Metronidazole | Amoxicillin-clavulanic acid | Single dose |
| Appendectomy | |||
| Ileostomy/colostomy closure | |||
| Biliary tract | Cefazoline | Clindamycin + Gentamycin | Single dose |
| Omphalocoele/gastroschisis repair with or without mesh | Cefazoline | Ampicillin + Gentamycin | Single dose or ≤24 h after closure |
| Closure with silo | |||
| Surgery without visceral involvement | None | ||
| Herniorrhaphy | |||
| Liver biopsy | |||
| Urological | |||
| Vesicoureteral reflux, pyeloplasty, ureterostomy | Cefazoline | Clindamycin + Gentamycin | Single dose |
| Cystoscopy with manipulation | |||
| Urethral valves | |||
| Hypospadias | |||
| Nephrectomy | |||
| Ureterocoele puncture | |||
| Cystoscopy without manipulation | None | ||
| Testicular torsion, orchidopexy, circumcision | |||
| Plastic surgery | |||
| With prosthetics, involving a large surface area of skin or of long duration | Cefazoline | Clindamycin o Vancomycin | Single dose (if risk of contamination, continue for 24 h) |
| Cutaneous or soft-tissue tumours with necrosis | |||
For most interventions, a single dose of an antibiotic with a long enough half-life to maintain antimicrobial activity throughout the procedure is sufficient.
A second dose should be administered during surgery if the duration of the procedure is greater than twice the half-life of the administered antibiotic or than 4 h or if the patient experiences profuse bleeding (>20 mL/kg of body weight). Outside these specific situations, prolonging treatment does not offer any benefits, even in high-risk gastrointestinal surgery.40
AdministrationIt is important to take into account the pharmacokinetics of the agent to achieve and maintain adequate serum concentrations before the surgical incision and through wound closure.
In the case of cefazoline, it should be administered 30−60 min before the start of the procedure, and in the case of vancomycin, the infusion should start 1–2 h before. The intravenous (IV) route is the route of choice for prophylaxis in newborns.
ScreeningIn patients undergoing cardiac, orthopaedic or neurological surgery, nasal screening for methicillin-resistant S. aureus (MRSA) is recommended, as is, in case of a positive result, the addition of one dose to vancomycin to the prophylaxis regimen (cefazoline), as prophylaxis with vancomycin alone has been associated with a higher proportion of SSIs by methicillin-sensitive S. aureus compared to cefazoline alone. Strategies such as intranasal mupirocin or the switch of the prophylactic antibiotic agent to vancomycin achieve a reduction in the prevalence of carriage, but their overall impact remains unclear.
Screening for multidrug-resistant microorganisms is not necessary before surgery. Colonised patients do not benefit from changes to the SAP. Implementation of appropriate isolation measures is recommended to prevent the spread of these pathogens.
Special cases- -
Patients with β-lactam allergy: treatment with vancomycin or clindamycin (after verification of susceptibility). Another antibiotic (gentamicin) can be added for gram-negative coverage as needed.
- -
Patients with humoral o cellular immunity deficiency: same prophylaxis regimen as immunocompetent patients.
- -
Patients who received antibiotherapy prior to surgery: they must still receive antibiotic prophylaxis before surgery to ensure adequate concentrations in plasma and tissue of antimicrobials active against the pathogens that may be present during surgery.
- -
Patients with unoperated cyanotic heart disease if they carry prosthetic material or have a previous history of infective endocarditis: prophylaxis against bacterial endocarditis.41,42
The correct and standardised diagnosis of health care-associated infections can help not only optimise treatment and improve outcomes, but also acquire real-world incidence data for the purpose of implementing preventive measures and evaluating their impact. The isolation of microorganisms in culture continues to be the gold standard of diagnosis, and antibiotherapy should be adjusted to the narrowest possible spectrum and discontinued in cases in which the culture results are negative.
Appendix. Members of the Standards Committee and the Commission on Neonatal Infection of the SENStandards Committee:
Héctor Boix (Unidad de Cuidados Intensivos Neonatales, Hospital Quironsalud, Barcelona, Spain), María Cernada (Servicio de Neonatología, Hospital Universitari i Politècnic La Fe, Valencia, Spain), María Gracia Espinosa Fernández (Unidad de Neonatología, Hospital Regional Universitario de Málaga, Málaga, Spain), Noelia González-Pacheco (Servicio de Neonatología, Hospital General Universitario Gregorio Marañón, Madrid, Spain), Alejandro Pérez-Muñuzuri (Ser[1]vicio de Neonatología, Hospital Clínico Universitario de Santiago, IDIS, Universidad de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain), M.ª Dolores Sánchez- Redondo (Unidad de Neonatología, Hospital Vir[1]gen de la Salud, Toledo, Spain), Ana Martín-Ancel (Servicio de Neonatología, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona, Spain), María Luz Couce (Servicio de Neonatología, Hospital Clínico Universitario de Santiago, IDIS, Universidad de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain).
Commission on Neonatal Infection:
Concepción de Alba Romero (Servicio de Neonatología, Hospital Universitario 12 Octubre, Spain), Belén Fernández-Colomer (Servicio de Neonatología, Hospital Universitario Central de Asturias, Oviedo, Spain), María Cernada (Servicio de Neonatología, Hospital Universitari i Politècnic La Fe, Valencia, Spain), María González-Lopez (Unidad de Neonatología, Hospital Materno-Infantil Regional de Málaga, Málaga, Spain), Elena Zamora Flores (Servicio de Neonatología, Hospital General Universitario Gregorio Marañón, Madrid, Spain), Laura Sánchez García (Servicio de Neonatología, Hospital Universitario La Paz, Madrid, Spain), Fátima Camba Longueira (Servicio de Neonatología, Hospital Vall d’Hebrón, Barcelona, Spain), M. Cruz López-Herrera (Servicio de Neonatología, Hospital Universitario de Cruces, Bilbao, Spain), Zenaida Galve Pradel (Servicio de Neonatología, Hospital Universitario Miguel Servet, Zaragoza, Spain), Carmen Ribes Bautista (Servicio de Neonatología, Hospital Vall d’Hebrón, Barcelona, Spain), Ana Alarcón Allen (Servicio de Neonatología, Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona, Spain), Ana Baña Souto (Servicio de Neonatología, Hospital Clínico Universitario de Santiago, IDIS, Universidad de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain).