Bartonella species are gram-negative intracellular bacteria that are responsible for 20%–30% of cases of endocarditis with negative blood culture results. The prevalence of Bartonella-related endocarditis in Spain is unknown, and in international studies1 it has been estimated that it corresponds to 1%–17% of the total cases of endocarditis. Within this genus, B. henselae has been associated with endocarditis in patients with a previous history of valvulopathy and contact with cats. The most frequently affected valve is the aortic valve, with the occasional report of a case with tricuspid valve involvement caused by an old pulmonary embolism.2
The method used most frequently for diagnosis is serologic testing, although real-time PCR can be used, allowing rapid detection of the bacterium in blood or valvular biopsy specimens.3
When it comes to treatment, most of the antibiotic agents that have proven efficacious, with the exception of the aminoglycosides, have a bacteriostatic effect, and several agents need to be combined. There is evidence of improved outcomes with administration of 1 aminoglycoside agent for at least 14 days.1 In patients with blood culture-negative endocarditis and suspected Bartonella infection, empirical treatment with gentamicin+ceftriaxone, with or without doxycycline, is considered appropriate. Cases with a confirmed diagnosis can be treated with a combination of doxycycline+gentamicin, considering the use of rifampicin, especially in patients with renal failure.
When it comes to the outcome of disease, most patients require valve replacement, and the mortality rate is 10%, with most deaths associated to delays in diagnosis.1
Since there are few published reports of paediatric cases in Spain, we believed that presenting this case would be relevant. We find this case particularly interesting due to the development of haemophagocytic syndrome (HPS), a complication that has rarely been described in association with B. henselae infection, especially in immunocompetent patients.
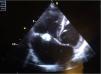
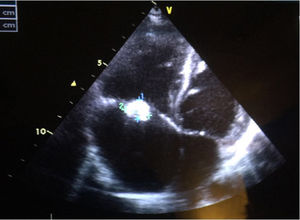
We present the case of a boy aged 10 years from a rural area in Morocco with no previous history of heart disease that reported intermittent fever, asthenia and shortness of breath with low-level exertion of 2 months duration. The physical examination evinced extreme weakness, moderate malnutrition, hepatosplenomegaly with the edge of the liver 3 finger breadths below the costal margin, petechiae in the lower extremities and grade VI/VI systolic murmur heard in all locations. The relevant findings of blood tests were anaemia (haemoglobin [Hb], 8.8g/dL) and light elevation of acute phase reactants (C-reactive protein, 59mg/L; erythrocyte sedimentation rate, 59mm). The echocardiographic examination revealed a perimembranous ventricular septal defect closed by a tricuspid aneurysm and multiple vegetations in the tricuspid valve, features compatible with native valve endocarditis (Fig. 1). The abdominal ultrasound scan revealed a splenic cyst 6mm in size with hyperechoic contents, compatible with an abscess. The chest CT scan revealed a triangular density with the base at the periphery of the left lower lobe suggestive of a small pulmonary infarction. The results of serial blood cultures were all negative, and B. henselae was detected through serologic testing (IgG 1/512). The evaluation of immune disorders was normal, including a negative result of HIV screening.
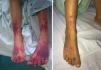
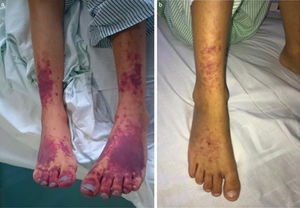
A 14-day course of ceftriaxone+gentamicin was initiated, and the regimen was switched to doxycycline+rifampicin upon receipt of the antibody test results. Three days later, coinciding with a complicated central line insertion, the patient exhibited clinical worsening with resurgence of fever, increased enlargement of liver and spleen (to 5 finger breadths) and development of purpuric lesions in the lower extremities (Fig. 2). The key findings of blood tests were coagulopathy, pancytopaenia (Hb, 7.3g/dL; white blood cells, 1200/mm3; platelets, 37000/mm3) and elevation of transaminase, ferritin and triglyceride levels, which fulfilled the diagnostic criteria for HPS. The patient was admitted to the PICU and stayed there for 3 days, during which he received boluses of methylprednisolone (30mg/kg/day), milrinone and diuretic agents for treatment of cardiac insufficiency. Blood culture results continued to be negative, and analysis of a bone marrow biopsy specimen found no evidence of haemophagocytosis. The patient showed improvement, both in symptoms and laboratory parameters, with normalisation of the blood panel values by day 5. The patient became asymptomatic after 8 weeks of antibiotherapy and 4 weeks of systemic steroid therapy. Although he had tricuspid regurgitation and vegetations continued to be evident on imaging, the patient was haemodynamically stable with adequate ventricular function, so the decision was made not to pursue heart surgery and instead closely monitor the patient from the cardiology department.
Infection, especially by herpesviruses, is the most frequent cause of secondary HPS.4B. henselae is an exceptional cause of HPS that has been described mostly in immunocompromised patients (transplant recipients or HIV+individuals), with no cases reported in association with endocarditis.5,6
Haemophagocytic syndrome is characterised by the uncontrolled activation and nonmalignant proliferation of macrophages and T cells associated with a massive release of cytokines. The diagnostic criteria endorsed by the Haemophagocytic Lymphohistiocytosis (HLH) study group, reviewed in 2004, continue to be relevant. In many cases, especially in primary forms of disease and in the initial stages, haemophagocytosis may not be evident in bone marrow biopsies.4
Given the poor prognosis of this disease, where mortality may reach up to 30%, it is important to remain alert for the development of HPS in the context of infection with clinical worsening and/or multiple organ involvement. Although treatment of the underlying disease is essential, some patients may require boluses of steroids or even immunosuppressant drugs, whose early administration may have a significant impact on outcome.
Please cite this article as: Núñez Cuadros E, Cabrera del Moral A, Jiménez Hinojosa JM, Leiva Gea I, Conejo Muñoz L. Síndrome hemofagocítico secundario a endocarditis por Bartonella henselae. An Pediatr (Barc). 2018;89:119–120.