Bronchopulmonary dysplasia (BPD) is the most common complication of preterm birth, and remains a major problem in paediatric pulmonology units. The decision of discharging from the Neonatal Unit should be based on a thorough assessment of the condition of the patient and compliance with certain requirements, including respiratory and nutritional stability, and caregiver education on disease management. For proper control of the disease, a schedule of visits and complementary tests should be established prior to discharge, and guidelines for prevention of exacerbations and appropriate treatment should be applied.
In this paper, the Working Group in Perinatal Respiratory Diseases of the Spanish Society of Pediatric Pulmonology proposes a protocol to serve as a reference for the follow up of patients with BPD among different centres and health care settings.
Key factors to consider when planning discharge from the Neonatal Unit and during follow up are reviewed. Recommendations on treatment and prevention of complications are then discussed. The final section of this guide aims to provide a specific schedule for follow-up and diagnostic interventions to be performed in patients with BPD.
La displasia broncopulmonar (DBP) es la secuela más prevalente del recién nacido pretérmino, y sigue suponiendo un motivo frecuente de consulta en las unidades de Neumología Pediátrica. La decisión del alta de la unidad neonatal debe apoyarse en una valoración exhaustiva de la situación clínica del paciente y en el cumplimiento de unos requisitos, que incluyen la estabilidad respiratoria y nutricional, y la instrucción a los cuidadores en el manejo domiciliario. Para un control adecuado de la enfermedad, es necesario que quede establecido, previamente al alta, un calendario de visitas y de exploraciones complementarias, y deben aplicarse las pautas de prevención de exacerbaciones y el tratamiento apropiados. El concepto de DBP como enfermedad multisistémica es fundamental en el seguimiento de los pacientes y debe ser tenido en cuenta para un buen control de la enfermedad.
En este documento, el Grupo de Trabajo de Patología Respiratoria Perinatal de la Sociedad Española de Neumología Pediátrica propone un protocolo que sirva como referencia para unificar el seguimiento de los pacientes con DBP entre los diferentes centros y ámbitos asistenciales.
Se revisan los aspectos a tener en cuenta en la evaluación previa al alta de la Unidad Neonatal y las principales complicaciones durante el seguimiento. Seguidamente, se detallan las recomendaciones en materia de tratamiento de la enfermedad y prevención de complicaciones, los controles tras el alta y su cronología.
Bronchopulmonary dysplasia (BPD) is a chronic pulmonary disease caused by impaired pulmonary and vascular development in which multiple perinatal factors are at play.1,2 It affects very low birth weight preterm newborns (PTNBs), especially those less than 1000g, and is the most frequent cause of respiratory morbidity in this population.
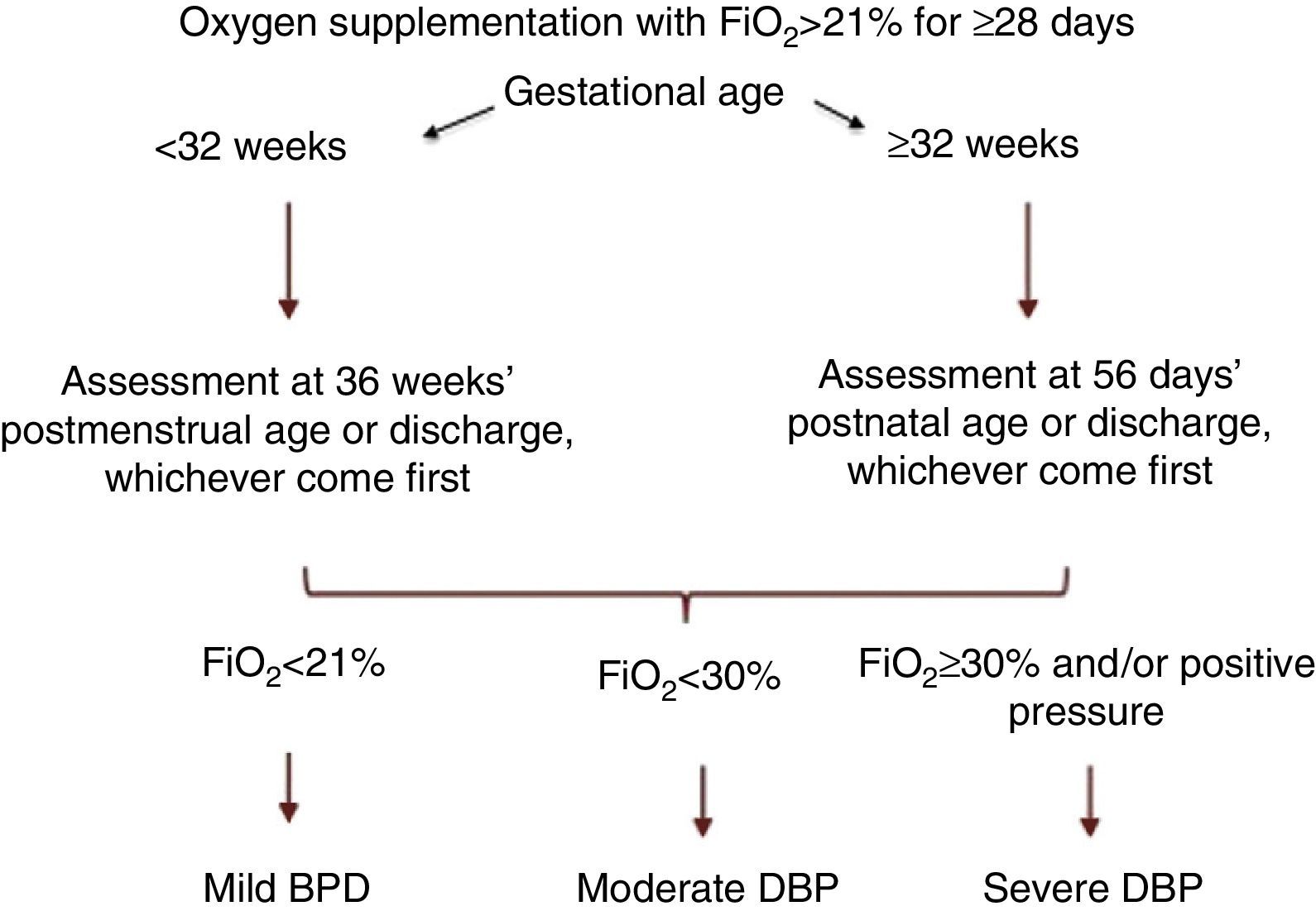
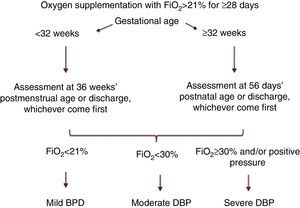
A consensus at the National Institute of Child Health and Human Development (NICHD) workshop proposed a definition of BPD as the need of supplemental oxygen (O2) by PTNBs for at least 28 days, and its classification as mild, moderate or severe based on O2 and ventilatory support requirements in a subsequent assessment3 (Fig. 1). Recently, the Sociedad Española de Neonatología (Spanish Society of Neonatology) revised the diagnostic criteria for BPD,4 recommending the addition of the physiological oxygen reduction test to the existing classification5 (Table 1).
BPD classification, NICHD consensus.1
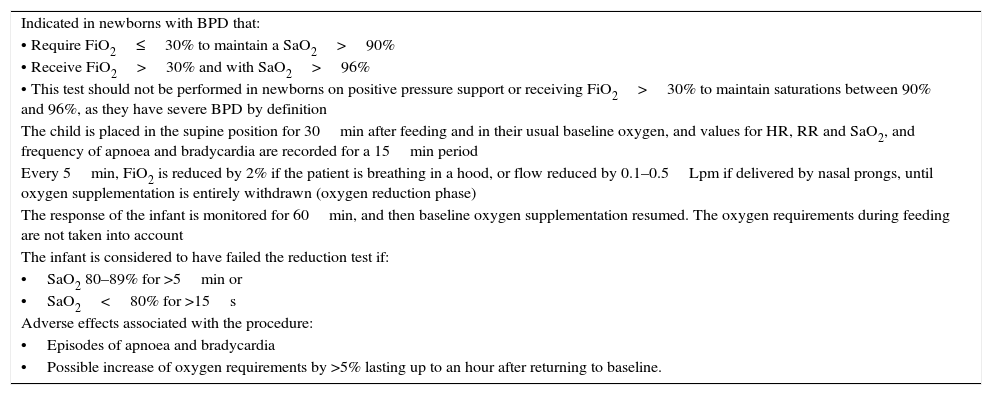
Oxygen reduction test for the physiologic definition of BPD.
| Indicated in newborns with BPD that: |
| • Require FiO2≤30% to maintain a SaO2>90% |
| • Receive FiO2>30% and with SaO2>96% |
| • This test should not be performed in newborns on positive pressure support or receiving FiO2>30% to maintain saturations between 90% and 96%, as they have severe BPD by definition |
| The child is placed in the supine position for 30min after feeding and in their usual baseline oxygen, and values for HR, RR and SaO2, and frequency of apnoea and bradycardia are recorded for a 15min period |
| Every 5min, FiO2 is reduced by 2% if the patient is breathing in a hood, or flow reduced by 0.1–0.5Lpm if delivered by nasal prongs, until oxygen supplementation is entirely withdrawn (oxygen reduction phase) |
| The response of the infant is monitored for 60min, and then baseline oxygen supplementation resumed. The oxygen requirements during feeding are not taken into account |
| The infant is considered to have failed the reduction test if: |
| •SaO2 80–89% for >5min or |
| •SaO2<80% for >15s |
| Adverse effects associated with the procedure: |
| •Episodes of apnoea and bradycardia |
| •Possible increase of oxygen requirements by >5% lasting up to an hour after returning to baseline. |
Source: Sánchez-Luna et al.4
Bronchopulmonary dysplasia is associated with longer lengths of stay, a higher incidence of respiratory and cardiovascular problems in the first two years of life, and neurodevelopmental delays and growth retardation.6 Children with a history of BPD may continue to have impairments in lung function through adolescence and adulthood.7
These patients require a close follow-up, including an appropriate assessment before discharge from the neonatal unit, the scheduling of follow-up visits and any pertinent diagnostic tests, and recommendations on the subject of treatment and prevention. In this regard, the Grupo de Trabajo de Patología Respiratoria Perinatal de la Sociedad Española de Neumología Pediátrica (Working Group on Perinatal Respiratory Pathology of the Spanish Society of Pulmonology [GTPRP-SENP]) has developed a protocol to guide specialists in charge of monitoring children with BPD.
Pre-discharge assessmentIt includes the following areas:
Oxygen therapyOnce the risk period for retinopathy has passed, oxygen therapy is indicated when the SaO2 on room air is 92% or less. It should be considered in patients with a SaO2 between 93% and 96% if there are signs of chronic lung disease and growth retardation despite adequate nutrition, and in patients with pulmonary hypertension (PH).
Imaging testsChest computed tomography (CT) is the most sensitive test for the detection of lesions secondary to BPD. The characteristic findings of BPD are multifocal decreased attenuation, linear and subpleural opacities, bronchial wall thickening, bullae and emphysema, although no radiological pattern serves as a prognostic factor.8 High-resolution CT with four to six slices per test is recommended to minimise radiation exposure. Due to the exposure to radiation and to the use of sedation by many medical facilities, we recommend reserving this method for cases in which it could provide information relevant to the management of the patient.
Pulmonary hypertension screeningThe prevalence of PH in children with BPD ranges between 18% and 43%.9 Echocardiography is the recommended method for screening for PH. It should be performed in patients with oxygen dependence at 36 weeks of postmenstrual age or at 2 months of life (or in case of clinical worsening or increased oxygen or ventilatory support requirements),10 and in patients with a history of intrauterine growth restriction and insufficient weight gain.
In some facilities, patients that require pulmonary vasodilators undergo cardiac catheterisation for confirmation of PH. We believe that this invasive strategy should be reserved for severe cases that do not respond to vasodilator therapy or when cardiovascular anomalies are suspected.10,11
Blood gasesIn severe cases, arterial blood gas analysis may need to be conducted prior to discharge to assess ventilation, although due to its invasiveness, blood gases are usually measured in capillary samples. Some degree of hypercapnia is commonly found.
AirwayIt is recommended that a fibrobronchoscopy is performed in patients treated with long-term mechanical ventilation (MV) or with a tracheostomy to rule out granulomas or tracheal or subglottic stenosis secondary to long-term intubation or tracheostomy tube dwelling. Fibrobronchoscopy is also recommended if there is suspicion of laryngomalacia or tracheobronchomalacia.
Respiratory functionIts assessment is restricted to specialty care facilities and it is still not part of routine evaluations due to its complexity, the lack of reference values and the need for sedation. The technique that offers the most information is rapid thoracoabdominal compression at tidal volume (maximal expiratory flow volume at functional residual capacity) and raised volume (forced vital capacity and forced expiratory volume). Plethysmography analyses the functional residual capacity, the total lung capacity and the residual volume. The analysis of breathing at tidal volume and pulmonary mechanics may also detect changes in respiratory status, although these tests are less sensitive.The results demonstrate a pattern of airflow obstruction with air trapping that tend to persist in time.12
Nutritional assessmentThe energy expenditure of patients with BPD is at least one third greater than in the rest of the preterm population, so these patients need a higher energy intake. Fluid overload must be avoided, with fluids provided only in the amount needed to ensure adequate diuresis.13 In patients in whom feeding is associated with increased respiratory effort or who fail to thrive, the option of nasogastric (NG) intubation and feeding must be assessed. If long-term NG feeding is likely or there are swallowing abnormalities that may facilitate pulmonary aspiration, the possibility of a gastrostomy should be evaluated.
Neurological assessmentThe neurology department should assess these patients prior to discharge and determine whether they require follow-up by a rehabilitation team.
Current pharmacological agents and discharge prognosisRefer to BPD treatment further down.
ImmunoprophylaxisPassiveThe use of palivizumab is recommended in children younger than one year with BPD. In the second year of life, prophylaxis may only be considered in children that have required treatment (oxygen therapy, bronchodilators, corticosteroids or diuretics) in the six months preceding the start of the RSV season.14 Prophylaxis is to be administered intramuscularly at doses of 15mg/kg of body weight once a month during the RSV season.
ActiveVaccination in the PTNB must be reinforced by the vaccination against influenza and pertussis of all individuals in the immediate environment of the patient (including siblings that have not been fully vaccinated with the DTaP) at least two weeks before direct contact. It is recommended that household members be vaccinated against influenza in the first two years of life of the PTNB.15
Vaccination against influenza is recommended in all children with BPD starting at age 6 months. The child will be vaccinated with the trivalent inactivated formulation authorised for that current year, receiving two 0.25mL doses one month apart the first year, and a single dose in subsequent years.
Invasive pneumococcal infections are especially frequent in PTNBs less than 32 weeks of gestational age. Thus, it is important that they receive the 13-valent pneumococcal conjugate vaccine. Although the levels of antibodies of PTNBs are lower than in children born to term, most reach levels above those considered protective against invasive pneumococcal disease.
Discharge criteriaThe timing of discharge must be determined on a case-to-case basis and coordinated by the entire interdisciplinary team. A series of criteria must be met16 (Table 2). It is essential that healthcare providers train caregivers, and that caregivers become progressively involved in caring for the infant (Table 3).
Criteria for hospital discharge.
| Satisfactory weight gain |
| Growth chart showing a sustained pattern of weight gain in the weeks prior to discharge. The use of artificial formulas with high-energy content is often necessary (100kcal/100ml). If breastfeeding is possible, it should be alternated and fortified with these formulas so that the child does not forego the benefits of breast milk. |
| Cardiovascular and respiratory stability |
| In the absence of recent changes to medication or supplemental oxygen flow, verify stable oxygenation in patient and the absence of respiratory exacerbations or episodes of apnoea or bradycardia |
| Ability to coordinate suckling, swallowing and breathing |
| The child must be able to feed by suckling without experiencing significant desaturation or choking episodes. If this is not the case, the use of NG intubation can be considered |
| Confirmation of immunisation status |
| Education of family members |
| Family members must be trained on basic CPR techniques and be provided with a self-inflating bag, face mask and tubing for connecting to the oxygen source. They must receive verbal and written information on the disease and its implications, natural history, signs of decompensation and strategies for preventing infection, especially by RSV |
| Social aspects |
| Social services must assess the situation of the family, process the paperwork to obtain the financial assistance needed by the family to the extent possible, and schedule home visits. The family must be provided with the necessary resources to guarantee adequate patient care at home and transport to medical appointments at the hospital |
| Discharge summary |
| Physical examination. Vital signs. SaO2 on room air and on FiO2 |
| Report of the last radiological examination and capillary blood gas test |
| Prescribed medication with dosage, schedule, and routes of administration. Oxygen therapy: prescribed device and flow. Phone number of device distributor |
| Nutrition: formula, preparation, volume and interval between feedings |
| Document the dates of administration of palivizumab and vaccines |
| Clear information on the schedule of appointments with all the specialists involved, trying to make them coincide. Ideally, care would be provided by an interdisciplinary team |
| Submission of the report to the primary care paediatrician must be attempted, and an appointment with primary care scheduled within the next 48–72h |
Training that caregivers must receive from the healthcare staff prior to hospital discharge.
| Secretion aspiration, placement of nasal prongs and management of tracheostomy if the patient has one, including changing and unblocking tubes |
| Feeding the infant (suckling, catheter or gastrostomy). Ability to provide supplemental oxygen during feeding in case of fatigue. Notions of postural measures to alleviate reflux or prevent it during sleep, if relevant |
| Drug dosage and delivery (oral solutions, inhalants). The regimen will be simplified as much as possible, trying to discontinue any medications that are not strictly necessary |
| Handling of oxygen therapy devices, nebulisers and pulse oximeter and understanding of device alarms. Preferably, caregivers will receive the equipment they will use at home ahead of time so they can familiarise themselves with it. If respiratory support is necessary, caregivers must be fully trained in handling the equipment prior to discharge, and subsequent follow-up by a home care team is required |
Respiratory diseases are most frequent, but comorbidity may also include nutritional, neurologic and cardiovascular disorders.17 The follow-up team must comprise multiple specialists to assist in the adequate physical and functional development of the infant.
Respiratory diseaseRespiratory morbidity is common in the first two years of life. It can include a broad range of disease, from severe cases requiring intensive care and oxygen therapy to cases that are completely asymptomatic. Patients may need oxygen therapy for months and frequent hospital care.17 They may suffer a slow and progressive deterioration in respiratory status, or have acute exacerbations that are almost always associated with viral infections or bronchial hyperresponsiveness. It is important to take preventive measures against deterioration and to detect it early. Food refusal, feeding fatigue, increases in respiratory rate and retractions are warning signs. Factors that may contribute to deterioration include fluid overload and cardiac decompensation (cor pulmonale), characteristic of severe cases of BPD. Measures must be taken to prevent infection by RSV in the community18 and to avoid potentially contagious environments such as childcare centres or scheduling hospital admissions for surgery during epidemic seasons.
If respiratory manifestations do not improve with standard treatment, gastroesophageal reflux (GER) should be ruled out, as it is more frequent in these patients than in other preterm infants, and may be exacerbated by air trapping. The risk of GER increases with theophylline use, NG intubation, and performance of gastrostomy prior to surgical correction of the reflux.
Growth disordersNutritional care is a key factor in the prevention and subsequent management of BPD.19 These children usually have inadequate growth, which may result from undernutrition, suboptimal oxygenation, and increased energy expenditure.
It is essential that growth parameters be strictly monitored for at least the first year of life by means of specific follow-up programmes.
Children with BPD often feed poorly due to anorexia or fatigue resulting from breathing difficulties. If the child shows poor weight gain, nutrient intake and changes in feeding must be assessed, and parent cooperation and ruling out GER must be considered. On the other hand, rapid growth increases the risk of obesity and insulin resistance, so the goal is not to achieve weight gain rapidly, but for it to correspond to an increase in lean mass.
Slow weight gain is closely related to respiratory impairment. Marginal hypoxaemia has been proven to cause growth retardation, so adequate oxygenation must be achieved.
Neurodevelopmental impairmentNeurodevelopmental impairment is more frequent in children with a history of BPD than in other PTNBs. The risk increases with prolonged MV, severe intraventricular haemorrhage (grade 3–4) and discharge past 43 weeks’ postmenstrual age.20 The impairment may affect visual and auditory perception, language, memory, the ability to learn and motor function. The prevalence of attention deficit disorder is higher in children with BPD.21 In addition, an association has been found between the early administration of systemic corticosteroids for treatment of BPD and long-term neurodevelopmental impairment.20
Cardiovascular disordersVascular hypoplasia and damage to the pulmonary microvasculature associated with BPD can lead to PH. The diagnosis is usually made after 2 months of life when the infant has already been discharged. Thus, PH screening programmes should include two or three echocardiograms in the first year of life, and additional echocardiograms before and after supplemental oxygen is withdrawn.11
Children with BPD may develop arterial hypertension, the cause of which remains unclear.22 It typically starts between the second and fourth months following discharge, is usually mild and responds well to treatment. Arterial blood pressure should be measured periodically during the follow-up.
Left ventricular hypertrophy has been reported in some patients, sometimes in association with the use of dexamethasone.
Treatment of bronchopulmonary dysplasiaPharmacotherapy after dischargeMedication is generally used to manage respiratory symptoms, although there is not a broad consensus on the drugs to be used once the severe stage of the disease has passed.23 The treatment should be determined on a case-to-case basis based on respiratory manifestations, supplemental oxygen requirements and growth outcomes. Monitoring the treatment is important to establish its duration and watch for potential side effects.
Inhaled bronchodilatorsInhaled bronchodilators should only be used if an acute episode of airway obstruction is suspected and the patient shows a good response to therapy.24
The most commonly used bronchodilators are short-acting beta2 agonists with the same dosage and forms of administration used in patients with symptoms of bronchospasm. These drugs may cause a paradoxical response in children with associated tracheobronchomalacia.
Anticholinergic agents (ipratropium bromide) are weaker bronchodilators, and while their use is not recommended in children with BPD they can be administered following the guidelines for the treatment of asthma attacks.24
Inhaled corticosteroidsThere is insufficient evidence of their effects on lung development or the management of airway obstruction, so they should be prescribed with caution.23,25 They may be useful for preventing recurrent episodes of wheezing following the guidelines for the treatment of asthma.
DiureticsThe use of diuretics is indicated in hypoxaemic patients with pulmonary oedema, or in case of severe lung disease with disturbed fluid homeostasis.
Furosemide is the most widely administered diuretic, but the existing evidence is insufficient to recommend its long-term use.26 Due to the potential associated risks (alkalosis, hyponatraemia and hypokalaemia; ototoxicity; nephrocalcinosis; cholelithiasis; osteopaenia), it is recommended that it be administered every other day for short periods of time.25,26 If the patient needs long-term diuretic therapy, the combination of thiazide and spironolactone is a reasonable alternative for minimising side effects under strict electrolyte monitoring and control. Discontinuation will be considered when there is symptom improvement, PH has resolved, and oxygen requirements are low.
Treatment of pulmonary hypertensionGeneral measuresGeneral measures include the optimisation of the respiratory and nutritional statuses. Chronic or intermittent hypoxaemia can exacerbate PH. Thus, the SaO2 must be maintained above 94% or 95%. The presence of comorbidities such as GER, pulmonary aspiration and structural airway anomalies must be ruled out.9
Pulmonary vasodilatorsNo randomised controlled clinical trials have been conducted, and there is a dearth of long-term safety and efficacy data. Their use is recommended for the treatment of moderate to severe PH when previous therapeutic approaches have failed.
Diseases in which the use of vasodilators could be detrimental need to be ruled out (aortopulmonary collaterals, left ventricular dysfunction, pulmonary vein stenosis or intracardiac shunts).11 To this end, some authors suggest cardiac catheterization prior to treatment initiation. Others propose performance of an echocardiogram or a CT angiogram and reserving catheterization for patients that respond poorly to treatment or for therapeutic purposes.11,9
Inhaled nitric oxide is used in patients that require invasive ventilatory support. However, due to the logistics of long-term management with inhaled nitric oxide and its high cost, it is advisable to substitute other vasodilators whenever possible.9
Oral sildenafil is the drug used most widely for the treatment of PH associated with BPD.27 Treatment starts with 0.5mg/kg doses every 8h. If the patient does not develop systemic hypotension, the dosage can be titrated up to a maximum of 2mg/kg every 6h.In patients that do not respond to sildenafil, administration of nebulised iloprost may improve PH and oxygenation.28
Epoprostenol delivered intravenously by continuous infusion is the most effective drug against PH, but it can produce hypotension and exacerbate hypoxaemia by increasing pulmonary vasodilation in non-aerated regions. In severe cases, oral bosentan or subcutaneous treprostinil can be added to the regimen.9
Oxygen therapyThe main purpose of home oxygen therapy in children with BPD is treating chronic or intermittent hypoxaemia secondary to the disease. Supplemental oxygen improves weight gain,29,30 decreases airway resistance, increases lung compliance31 and reduces pulmonary hypertension.32 The incidence of sudden infant death syndrome and episodes of obstructive sleep apnoea is lower in children with oxygen saturations that remain above 93%.23
Indications for home oxygen therapyThe use of supplemental oxygen requires avoiding both hypoxia and oxygen toxicity. No clinical trials have been conducted to establish the SaO2 criteria on the basis of which to initiate or continue oxygen therapy. The current recommendations are based on reference values in healthy children and observational studies on the effects of hypoxia.33 Following the guidelines of neonatology units, it seems reasonable to apply the SaO2 range of 88–92%, used in the physiologic definition, as a threshold to discontinue oxygen supplementation in PTNBs at risk of developing retinopathy. Once they reach the full-term gestational age and retinal vascular maturity, supplemental oxygen will be administered in the amounts needed to achieve a SaO2 of 93% or above, or of 95% and higher in patients with a documented history of PH or growth retardation.34
The equipment that is usually recommended is liquid oxygen tanks along with a backpack for travel. Oxygen concentrators provide an alternative for patients with minimal but long-term oxygen requirements. These devices can produce flow rates of 0.1–4Lpm. Usually, the patient requires a low flow rate (0.5–1Lpm) that is delivered by means of nasal prongs. At these flow rates, humidification is not necessary, except in patients with a tracheostomy.
MonitoringA pulse oximeter is usually provided to monitor and titrate home oxygen requirements. This device offers the family an early warning system, although this method has the downside of motion artefacts.35 Nevertheless, it allows parents or caregivers to have access to continuous information and it reduces the number of medical visits and calls. It also helps guide decisions to increase oxygen flow rates or about the need to go to a healthcare facility.36 If the patient improves, the flow rate can be gradually decreased under close watch, leading to shorter durations of home oxygen therapy.
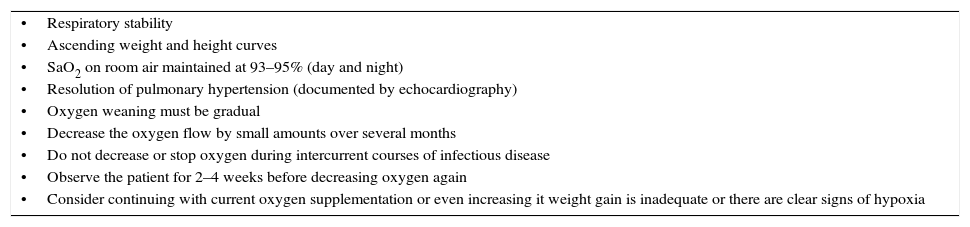
Oxygen removalThe factors presented in Table 4 must be taken into account. After ascertaining that the SaO2 remains stable on room air (93–95%), it is advisable that the oxygen therapy devices are kept in the home for a while, especially during epidemic seasons for respiratory infections.
Factors to consider in the discontinuation of home oxygen therapy.
| •Respiratory stability |
| •Ascending weight and height curves |
| •SaO2 on room air maintained at 93–95% (day and night) |
| •Resolution of pulmonary hypertension (documented by echocardiography) |
| •Oxygen weaning must be gradual |
| •Decrease the oxygen flow by small amounts over several months |
| •Do not decrease or stop oxygen during intercurrent courses of infectious disease |
| •Observe the patient for 2–4 weeks before decreasing oxygen again |
| •Consider continuing with current oxygen supplementation or even increasing it weight gain is inadequate or there are clear signs of hypoxia |
Along with postural changes, it aids the mobilisation of secretions and bronchial drainage in children with persistent atelectasis or thick secretions or on long-term MV. Different techniques are used, such as vibration, pressure on the chest and abdomen, exercises for pulmonary re-expansion, positioning for bronchial drainage, soft percussion, forced coughing and stimulation of the Vojta chest area.37
For home care, it is recommended that these methods continue to be applied in patients with severe disease, as chest physical therapy seems to have beneficial effects.38
Nutritional supportThe main purpose of nutritional support is to optimise growth and development. The feeding methods and composition of enteral formulas are subject of debate. Formulas specifically designed for premature infants must be used in order to deliver the right amount of calories, especially in the early months of life, and at times it is necessary to supplement with carbohydrates or fats. The use of mineral and vitamin supplements, required by all premature infants, must also be considered with care.23
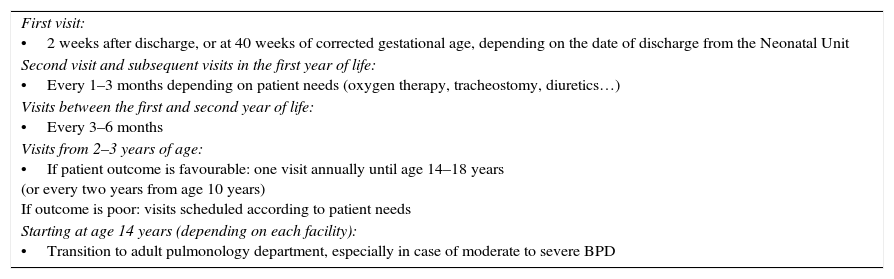
Visit schedule. Care protocolThis section summarises the post-discharge follow-up care plan for children with BPD after hospital discharge proposed by the GTPRP-SENP. It applies to all children that have received a BPD diagnosis, including those with mild disease. Table 5 shows the recommended visit schedule. This schedule is proposed for guiding purposes and is to be modified by the physician in charge based on the needs of each patient. Table 6 describes the clinical follow-up, and Table 7 provides a summary of the diagnostic tests to consider during the follow-up period.
Recommended appointment timeline for patients with BPD.
| First visit: •2 weeks after discharge, or at 40 weeks of corrected gestational age, depending on the date of discharge from the Neonatal Unit |
| Second visit and subsequent visits in the first year of life: •Every 1–3 months depending on patient needs (oxygen therapy, tracheostomy, diuretics…) |
| Visits between the first and second year of life: •Every 3–6 months |
| Visits from 2–3 years of age: •If patient outcome is favourable: one visit annually until age 14–18 years (or every two years from age 10 years) If outcome is poor: visits scheduled according to patient needs |
| Starting at age 14 years (depending on each facility): •Transition to adult pulmonology department, especially in case of moderate to severe BPD |
Clinical assessments at different visits.
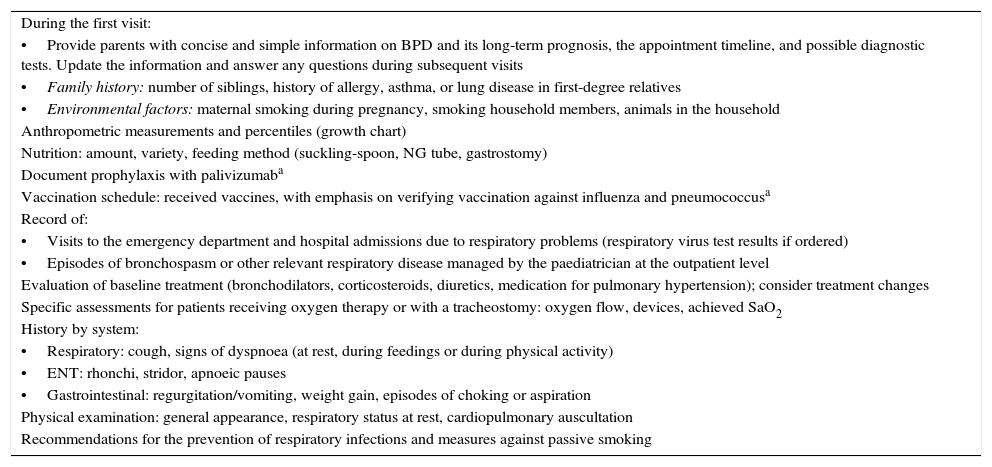
| During the first visit: |
| •Provide parents with concise and simple information on BPD and its long-term prognosis, the appointment timeline, and possible diagnostic tests. Update the information and answer any questions during subsequent visits |
| •Family history: number of siblings, history of allergy, asthma, or lung disease in first-degree relatives |
| •Environmental factors: maternal smoking during pregnancy, smoking household members, animals in the household |
| Anthropometric measurements and percentiles (growth chart) |
| Nutrition: amount, variety, feeding method (suckling-spoon, NG tube, gastrostomy) |
| Document prophylaxis with palivizumaba |
| Vaccination schedule: received vaccines, with emphasis on verifying vaccination against influenza and pneumococcusa |
| Record of: |
| •Visits to the emergency department and hospital admissions due to respiratory problems (respiratory virus test results if ordered) |
| •Episodes of bronchospasm or other relevant respiratory disease managed by the paediatrician at the outpatient level |
| Evaluation of baseline treatment (bronchodilators, corticosteroids, diuretics, medication for pulmonary hypertension); consider treatment changes |
| Specific assessments for patients receiving oxygen therapy or with a tracheostomy: oxygen flow, devices, achieved SaO2 |
| History by system: |
| •Respiratory: cough, signs of dyspnoea (at rest, during feedings or during physical activity) |
| •ENT: rhonchi, stridor, apnoeic pauses |
| •Gastrointestinal: regurgitation/vomiting, weight gain, episodes of choking or aspiration |
| Physical examination: general appearance, respiratory status at rest, cardiopulmonary auscultation |
| Recommendations for the prevention of respiratory infections and measures against passive smoking |
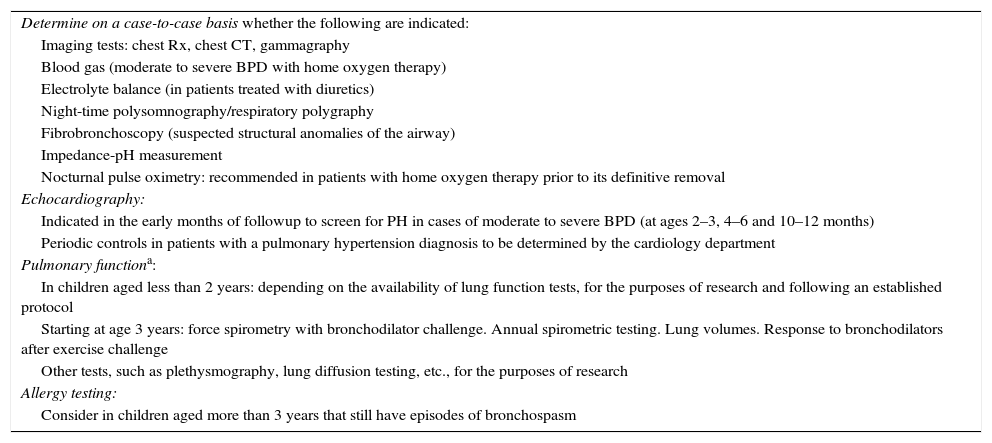
Diagnostic tests performed during the followup of patients with BPD.
| Determine on a case-to-case basis whether the following are indicated: |
| Imaging tests: chest Rx, chest CT, gammagraphy |
| Blood gas (moderate to severe BPD with home oxygen therapy) |
| Electrolyte balance (in patients treated with diuretics) |
| Night-time polysomnography/respiratory polygraphy |
| Fibrobronchoscopy (suspected structural anomalies of the airway) |
| Impedance-pH measurement |
| Nocturnal pulse oximetry: recommended in patients with home oxygen therapy prior to its definitive removal |
| Echocardiography: |
| Indicated in the early months of followup to screen for PH in cases of moderate to severe BPD (at ages 2–3, 4–6 and 10–12 months) |
| Periodic controls in patients with a pulmonary hypertension diagnosis to be determined by the cardiology department |
| Pulmonary functiona: |
| In children aged less than 2 years: depending on the availability of lung function tests, for the purposes of research and following an established protocol |
| Starting at age 3 years: force spirometry with bronchodilator challenge. Annual spirometric testing. Lung volumes. Response to bronchodilators after exercise challenge |
| Other tests, such as plethysmography, lung diffusion testing, etc., for the purposes of research |
| Allergy testing: |
| Consider in children aged more than 3 years that still have episodes of bronchospasm |
The authors have no conflict of interests to declare.
Please cite this article as: Pérez Tarazona S, Rueda Esteban S, Alfonso Diego J, Barrio Gómez de Agüero MI, Callejón Callejón A, Cortell Aznar I, et al. Protocolo de seguimiento de los pacientes con displasia broncopulmonar. An Pediatr (Barc). 2016;84:61.