Food protein-induced enterocolitis syndrome (FPIES) is a gastrointestinal syndrome due to a non-IgE mediated food hypersensitivity. The most common triggers are cow's milk and soy. Fish is one of the most frequently reported causes in Spain.
The objective of this study is to describe the clinical characteristics of patients diagnosed with (FPIES) in a paediatric allergy clinic.
Material and methodsA retrospective descriptive study was carried out by reviewing medical records of patients diagnosed with FPIES in the Paediatric Allergy Unit of the Miguel Servet Children's Hospital from the years 2007 to 2017.
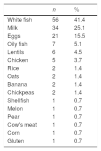
ResultsA total of 135 patients were diagnosed during the study period, of whom 45% were male and 55% were female. The mean age at diagnosis was 11±1.5 months and the mean age of improvement was 2 years and 6 months±2.5 years (n=83). A personal history of atopy was observed in 31.9%. The main trigger foods were: white fish (41.4%), cow's milk (25.1%), and egg (15.5%). A conversion to IgE-mediated allergy was seen in 4.4% of patients.
There was vomiting in 81.5% of the cases, with a mean of 1.75±1.1h of latency, as well as diarrhoea in 41.5%, with a mean of 7.86±15.16h of latency, and decline in 30.4% with a mean latency of 3.81±11.57h.
DiscussionIn our series, the most frequent trigger of the FPIES was fish. It was manifested mainly by late vomiting and a tolerance was reached mostly at 2 years 6 months.
La enterocolitis inducida por proteínas de la dieta, o también conocida como food protein-induced enterocolitis syndrome (FPIES), es un síndrome gastrointestinal de hipersensibilidad alimentaria no mediada por IgE. Los desencadenantes más comunes son la leche de vaca y de soja. El pescado es una de las causas reportadas con más frecuencia en España.
El objetivo de esta investigación es describir las características clínicas de los pacientes diagnosticados de FPIES en nuestra consulta de alergología pediátrica.
Material y métodosEstudio descriptivo retrospectivo, realizado mediante revisión de historias clínicas de los pacientes diagnosticados con FPIES en la Unidad de Alergología Pediátrica del Hospital Infantil Miguel Servet desde 2007 a 2017.
ResultadosDesde enero de 2007 hasta diciembre de 2017 fueron diagnosticados 135 pacientes: 45% hombres y 55% mujeres. La edad media al diagnóstico fue de 11±1,5 meses y la edad media de superación 2 años y 6 meses±2,5 años (n=83). Un 31,9% presentó antecedentes personales de atopia. Los principales alimentos desencadenantes fueron: pescado blanco (41,4%), leche de vaca (25,1%) y huevo (15,5%). Un 4,4% presentó conversión a alergia IgE mediada.
El 81,5% de los casos presentó vómitos, con una media de 1,75±1,1horas de latencia; diarreas en un 41,5%, con una media de 7,86±15,16horas de latencia; decaimiento en el 30,4% con una media de 3,81±11,57horas de latencia.
DiscusiónEn nuestra serie el desencadenante más frecuente fue el pescado. Se manifestó principalmente por vómitos tardíos, y alcanzó una tolerancia en su mayoría hacia los 2 años 6 meses.
The European Academy of Allergology and Clinical Immunology has proposed a classification of food allergy based on the immune mechanisms involved, grouping allergies into IgE-mediated and non-IgE-mediated.1
Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE-mediated gastrointestinal hypersensitivity syndrome. It is considered a childhood disease, although it can also manifest in adults.2 The exact underlying pathophysiological mechanisms are unknown, but it has been proposed that T cell activation and TNF-α production play a role in local inflammation and increased permeability in the gut, which contribute to fluid shift and result in clinical manifestations such as pallor, hypoperfusion or hypothermia.3 The role of humoral immunity has also been investigated, but it is not clearly defined yet.4
The data on the prevalence of FPIES are scarce. In 2011, Katz et al. published a prospective study that found a cumulative incidence of cow's milk protein-induced FPIES of 0.34%, with a higher frequency in boys.5
Based on the current literature, the most common triggers of FPIES are cow's milk and soy, accounting for 50% of cases, although it can be triggered by a broad range of other foods, including fish, legumes or meat.6–9
The typical presentation is acute, with onset between 1 and 4h following ingestion of the culprit food, and characterised by repetitive vomiting, pallor, progressive lethargy, dehydration and hypovolaemic shock in up to 15% of cases.8,10 In some instances, diarrhoea develops 5–10h after ingesting the food.11 A chronic form has been described that is characterised by intermittent vomiting, episodes of diarrhoea and poor weight gain with progression to dehydration and hypovolaemic shock over a few days or weeks.
Tests for detection of specific IgE antibodies are usually negative, although a small percentage of patients may develop IgE-mediated allergy.5,8,12 There is widespread international consensus that the diagnosis of FPIES requires that a patient meet certain diagnostic criteria (1 major criterion and 3 or more minor criteria). The major criterion is vomiting 1–4h after ingestion of the suspect food without IgE-mediated allergic symptoms.
The minor criteria are: (1) a second (or more) episode of vomiting after eating the same suspect food; (2) repetitive vomiting 1–4h after eating a different food; (3) extreme lethargy; (4) marked pallor; (5) need for emergency care; (6) need for intravenous fluid support; (7) diarrhoea in 24h (usually 5–10h); (8) hypotension and (9) hypothermia. Another important aspect to consider is that acute FPIES reactions will typically resolve in full over a matter of hours and the patient should be asymptomatic and growing normally when the offending food is eliminated from the diet. When it comes to chronic FPIES, there are 2 distinct forms. The first one is the severe presentation, in which the offending food is eaten on a regular basis (for example, infant formula), manifesting with intermittent but progressive vomiting and diarrhoea, sometimes with dehydration and metabolic acidosis. In the mild presentation, the ingested doses of the problem food are lower (such as food allergens in breast milk) and cause intermittent vomiting and/or diarrhoea with poor weight gain but without dehydration or metabolic acidosis. The most important criterion for diagnosis of chronic FPIES is resolution of the symptoms in a few days following elimination of the offending food(s) and acute recurrence of symptoms when the food is reintroduced, with onset of vomiting in 1–4h and diarrhoea in 24h (usually 5–10h).13
Therefore, the most important diagnostic tool is a detailed history-taking,12,13 which is sufficient for diagnosis in the vast majority of patients. If the diagnosis is inconclusive, an oral food challenge is recommended for confirmation, an approach that is also used to determine whether the patient has outgrown FPIES. Children with a history strongly consistent with FPIES do not require an oral food challenge, as the risks of the challenge may outweigh its benefits.13 There are no laboratory markers or any other specific diagnostic tests for FPIES, although there are several tests that may be used to support the diagnosis or rule out other diseases.12,13
Acute presentations may be clearly compatible with other diseases, which may pose challenges in diagnosis. For example, a single episode associated with fever and contact with diseased individuals is suggestive of infectious gastroenteritis. Acute FPIES reactions usually resolve completely in a few hours, compared to the usual duration of several days characteristic of infectious gastroenteritis. When it comes to sepsis, fluid resuscitation is not effective in isolation, and requires additional treatment such as antibiotherapy. Necrotising enterocolitis usually presents in newborns and young infants with rapid disease progression, bloody stools, shock and detection of intramural gas in abdominal X-rays. Immune enteropathies (inflammatory bowel disease, autoimmune enteropathy or immunodeficiencies) are rare in infancy and are not usually associated with the ingestion of specific foods. Surgical emergencies (malrotation, volvulus, etc.) are not associated with specific food intake and present with evidence of obstruction in imaging tests.13
Treatment is based on the elimination of the trigger food from the diet, after which acute FPIES usually resolve within 4–12h; however, severe cases may require bowel test and intravenous fluid therapy.
Outcomes are usually favourable and most children develop tolerance to the food by age 3–5 years,8 although there are cases in which FPIES persists in adulthood.2,8 Spontaneous tolerance to foods such as cow's or soy milk tends to happen at earlier ages compared to solid foods.8,14 Complementary feeding in infants with FPIES should adhere to the guidelines that apply to any healthy infant in similar circumstances, except for the avoidance of the trigger food.12
At present, there are no biomarkers for FPIES or any treatment that accelerates its resolution, so further research is required to investigate and improve clinical manifestations in affected patients.
The aim of our study was to describe the clinical characteristics of patients given a diagnosis of FPIES in the real-world practice setting of a paediatric allergy clinic.
Material and methodsWe conducted a retrospective and descriptive study through the review of the health records of patients given a diagnosis of enterocolitis in the paediatric allergy clinic of our hospital between 2007 and 2017.
The inclusion criteria were: patients with manifestations compatible with enterocolitis (delayed repetitive vomiting [with onset at least 1h after ingestion of the suspect food], pallor, asthenia-lethargy and malaise and/or diarrhoea after ingestion of certain foods with negative skin prick tests and/or recurrence of symptoms on reintroduction of the suspect food in patients undergoing an oral food challenge).
The variables under study were the following: age, sex, personal and family history of atopy, type of feeding, trigger food, symptoms, conversion to IgE-mediated allergy to the same food, previous tolerance, diseases considered in the initial differential diagnosis (by the primary care paediatrician, in the emergency department or during hospitalisation in the ward), need for emergency care, observation and/or hospital admission, age of resolution/outgrowing of syndrome.
We performed the descriptive statistical analysis of demographic data using the software SPSS version 21. We have summarised continuous data (or quantitative data or data on an interval scale) as mean and standard deviation, specifying the value of the standard error if necessary.
ResultsFrom January 2007 to December 2017, a total of 135 patients received a diagnosis of FPIES; 45% were male and 55% female.
The mean age at onset was 11±1.5 months, with a range of 3 months to 10 years, and the mean age at which FPIES was outgrown was 2 years and 6 months±2.5 years, with a range of 2 months to 13 years and 10 months.
Of all patients, 68.1% had no personal history of atopy. Of the remaining patients, 26% had atopic dermatitis, 3% a diagnosis of IgE-mediated allergy to other foods and 2.2% a diagnosis of allergic asthma.
A family history of atopy in a first-degree relative was present in 55.6% of the patients.
We analysed the modality of feeding in the first 6 months of life, for which we were able to obtain information for 36 patients: 55.5% had been breastfed, 11% formula-fed and 33.5% received both breast milk and formula.
An oral food challenge was performed in 67.6% of patients to assess whether they had outgrown the allergy.
Most patients had FPIES in response to a single food. Table 1 presents the foods that triggered reactions in our sample. We found that 11.9% developed symptoms in response to several foods, and in this subset, 86% reacted to both white and oily fish.
Of the patients with FPIES induced by a single food, only 4.4% converted to IgE-mediated allergy to that food at a later time.
We analysed the most frequent manifestations in our patients. Vomiting occurred in 81.5%, with a mean latency period of 1.75±1.1h (range, 0–7h); diarrhoea in 41.5%, with a mean latency of 7.86±15.16h (range, 0–72h); asthenia in 30.4% with a mean latency of 3.81±11.57h (range, 0–72h) and pallor in only 14.1% with a mean latency of 2.04±0.84h (range, 1–4h).
In 38.5%, symptoms appeared the first time they ingested the culprit food. In 15.5%, the initial diagnosis was not FPIES, with the most frequent misdiagnosis being acute gastroenteritis. Seventeen percent of patients visited the emergency department before receiving the diagnosis of FPIES in our unit; of these patients, 8.9% were admitted to the observation unit in the emergency department for intravenous rehydration and 9.6% were admitted to hospital. All other patients were assessed by a primary care physician that made the referral to our unit for evaluation.
DiscussionWe present a study in which we analysed the clinical characteristics of paediatric patients with a diagnosis of FPIES managed in a paediatric allergy clinic.
In this study, we found a slight predominance of the female sex (55%), unlike other studies where the male sex predominated (60:40 ratio).3 Patients with FPIES triggered by cow's milk or soy usually had onset at an earlier age (<6 months) compared to patients with FPIES triggered by solid foods (6–12 months).13 The mean age at onset in our sample was 11±1.5 months, possibly because fish was the most frequent trigger.
Thirty percent of patients with FPIES developed disorders manifesting with atopy, such as atopic dermatitis, asthma or allergic rhinitis,3 with associated atopic dermatitis found in 9–57% of the total.6,14,15 Our findings were consistent with this, as 31.9% of our patients had a history of allergic disorders, which was atopic dermatitis in 26%.
The proportion of patients with a family history of atopy varies between publications, ranging between 20% and 77%.8 In our study, 55.6% of patients had a history of atopy in a first-degree relative.
The solid food involved most frequently was fish, and the liquid food involved most frequently was cow's milk, findings that were similar to those of Nowak et al.,8 who found the same results in Spanish and Italian cohorts, differing from other geographical regions. According to the authors of the international consensus guidelines for the diagnosis and management of FPIES, published in 2017, this difference could be explained by multiple factors such as differences in the case series under study, presence of atopic disease, breastfeeding, dietary practices and genetic factors that have yet to be discovered.13 In a multicentric study published in 2019, Díaz et al. found that the most frequent trigger food was cow's milk, followed by fish and eggs.9
In an Italian cohort of children with FPIES, 95% had been breastfed for a median of 4 months (range, 0.5–12) compared to a proportion of breastfed children of 47% in a large cohort in the United States.6 In our study, in the subset of patients for who we had data on this variable, 55.5% had been breastfed the first 6 months of life, 11% had been fed formula and 33.5% had received mixed feeding.
As for the latency period between ingestion of the food and the onset of symptoms, Katz et al. reported a range of 30min to 5.25h, with symptoms appearing after 2h in 60% of cases.5 In our case series, we found that the most frequent symptom, vomiting, appeared after a mean latency period of 1.75±1.1h (range, 0–7h). The existing literature identifies vomiting as the most frequent symptom of FPIES,9 with diarrhoea usually reported in 50% or fewer patients.6,11 Our findings were similar, with vomiting occurring in 81.5% of patients and diarrhoea in 41.5%.
In a study by Sopo et al., 47% of acute reactions with diarrhoea and 27% of reactions without diarrhoea required hospital admission and intravenous fluid therapy.14 Hypotension has also been reported in 5–77% of patients in several cohorts.6 We found that 17% of the patients in our sample required emergency care, 8.9% had to remain in observation in the emergency department to receive intravenous rehydration, and 9.6% required hospital admission.
All patients referred to our clinic had a presentation with acute onset following ingestion of a food, as patients with a chronic presentation are referred to paediatric gastroenterology clinics.
On the other hand, we ought to underscore that currently it is recommended that clinicians consider the possible coexistence of IgE-mediated allergy to the trigger food,13 as this condition may be associated with an increased probability of persistent illness.15 In our sample, 4.4% of patients with FPIES exhibited conversion to IgE-mediated allergy to the same food.
In our study, the mean age at which patients outgrew the allergy was 2 years and 6 months, with a range of 2 months to 13 years and 10 months. The reported mean age by which tolerance was developed was 35 months for legumes and 42 months for other solid foods (vegetables, fruits, meats). Studies in Spain and Italy have found that fish-induced FPIES is outgrown by 5.5 years.8 The study by Katz et al. found that of the total patients with non-IgE-mediated cow's milk allergy, 50% had developed tolerance by age 1 year, 75% by 18 months, 88.9% by 2 years and 94.4% by 30–36 months.5
We also found that most patients (88.1%) had FPIES induced by a single food, which was similar to the findings of Mehr et al. in the Italian and Spanish populations, with a percentage exceeding 80%.7 The findings of the study of Diaz et al. were also similar, as 84.2% of patients also had FPIES induced by a single food.9
Our study had the limitations intrinsic to a retrospective design. Since we included cases diagnosed over a period of 10 years without the application of clear criteria for each diagnosis during this whole time, there may have been misdiagnoses, leading to an erroneous case count. The fact that we also did not have complete data for all the patients is also a significant limitation.
Another weakness of our study is that oral food challenges are not performed in our paediatric allergy clinic to confirm diagnosis. This test is only used to confirm the resolution of FPIES, and therefore the diagnosis of FPIES in all patients with inconclusive manifestations may not have been accurate, which may have led to misdiagnosed or unconfirmed cases and an overestimation of the actual frequency of the disease, leading to unreliable findings, for instance, potentially calculating a very early age of resolution. There may be further bias in the age of resolution due to the missing data of patients who did not come to the clinic for the oral food challenge (32.4%) performed to establish resolution of FPIES.
In conclusion, the most frequent trigger in our patients was fish, followed in frequency by cow's milk and eggs. Most cases involved reactions to a single food, compared to cases with symptoms induced by 2 or more foods, highlighting the importance of avoiding dietary restrictions in complementary feeding that may hinder nutrition and adequate growth in children with FPIES.
The most important contribution of this study may be the presentation of data on a sizable case series, although some may have been misdiagnosed. Due to the latter, we consider it essential for clinicians to be knowledgeable of current international diagnostic criteria in order to improve the accuracy of diagnosis and the management of a disease that is still poorly diagnosed in our region.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez RI, Carrión SK, Aliaga Y, Calvo JB, Guallar MI. Nuestra experiencia en enterocolitis inducida por proteínas de la dieta en la consulta de alergología pediátrica. An Pediatr (Barc). 2020;92:345–350.
Previous presentation: This study was presented at the International Symposium of the Sociedad Española de Alergología e Inmunología Clínica, October 23–26, 2019.