Although changes in liver function tests can be non-specific in numerous clinical conditions, they can be the first sign of a potentially serious disease in an asymptomatic patient.
Material and methodsRetrospective cohort study, performed by reviewing the records of children of a reference hospital central laboratory with alanine aminotransferase enzyme (ALT) elevation during a 6-month aleatory period.
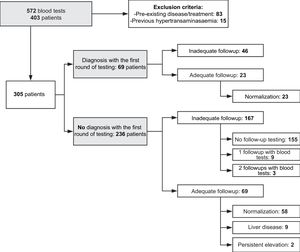
Results572 blood tests with serum ALT elevation corresponding to 403 patients have been assessed during the period studied. 98 patients were excluded for presenting abnormal liver test before the study period of comorbidity that could produce ALT elevation. The remaining 305 patients, 22.6% were diagnosed with a medical condition during the first blood test that explained the ALT elevation, although only 33.3% of them were followed up until verifying their normalisation. Final study sample consists of 236 patients with abnormal liver test without apparent liver disease. Adequate follow-up was found only in 29% of them. From this group, 9 patients (13%) were diagnosed with liver disease. The rest of the samples were not properly monitored. In patients with higher serum ALT levels, follow-up was early and more appropriate.
ConclusionsIn our area, most children without apparent liver disease are no properly monitored. Therefore, an opportunity to diagnosis and treat a potential liver disease was lost in a great number of children. All children with unexplained hypertransaminasaemia must be studied.
Las alteraciones del perfil hepático constituyen un hecho inespecífico propio de numerosas condiciones clínicas. Sin embargo, puede implicar la primera manifestación de una patología potencialmente grave en un paciente asintomático.
Material y métodosEstudio observacional retrospectivo que incluye todas las analíticas sanguíneas con elevación de alanino aminotransferasa (ALT) en pacientes pediátricos solicitadas en un sector sanitario en un período de 6meses.
ResultadosSe registraron 572 analíticas correspondientes a 403 pacientes. Se excluyeron 98 pacientes con hipertransaminasemia ya conocida o comorbilidad. De los 305 restantes, el 22,6% se diagnosticaron de patología asociada a hipertransaminasemia, y de estos, se comprobó normalización en el 33,3%. De los 236 pacientes con hipertransaminasemia sin justificar se realizó un seguimiento en el 29%, encontrando patología hepática en 9pacientes (13% del grupo). En el resto de la muestra no se comprobó analíticamente la evolución de las transaminasas ni la presencia de posible patología hepática. Los pacientes con cifras más elevadas se controlan mejor y antes que los que presentan cifras más bajas.
ConclusionesEn nuestra área, la mayoría de los niños sin enfermedad hepática aparente con hallazgo de ALT elevada no son adecuadamente controlados. Esto hace que se pierda una oportunidad única de diagnosticar y tratar precozmente una enfermedad hepática potencial en un gran número de niños. Todo niño con hipertransaminasemia inexplicada debe ser estudiado.
Abnormalities in the liver panel are among the most frequent changes detected in clinical practice at both the hospital and primary care levels. Routine performance of liver panels has evinced an increase in the incidence of abnormal serum levels of liver enzymes, either as a chance finding in asymptomatic patients or in the context of mild symptoms.1,2
The elevation of liver enzymes is a nonspecific finding present in many clinical conditions. However, it can also be the first manifestation of potentially severe liver disease in an apparently healthy patient or a secondary manifestation of a serious extrahepatic disease.1,3,4
The underlying causes of liver enzyme elevation are heterogeneous and differ broadly depending on the population. Although in adults it is most frequently associated with the use of alcohol or drugs, in the paediatric population it is predominantly associated with transient infectious diseases (local or systemic, viral or bacterial).2,3,5,6
The lack of universal reference values in children and their dependence on various factors (stage of development, sex, age, etc.) have prompted the performance in recent years of multiple studies that attempt to establish thresholds that vary between publications. On the other hand, there are no established clinical guidelines for the evaluation and follow-up of hypertransaminasaemia in paediatric practice.7–18
Nevertheless, it is remarkable that, despite the considerable significance of this condition, the general tendency at present is to perform an insufficient follow-up of these patients, globally and in our region in particular. Therefore, the aim of our study was to analyse the follow-up of patients with hypertransaminasaemia and without apparent liver disease, which has not been addressed previously in the paediatric literature.
Material and methodsWe conducted a retrospective, observational and descriptive study. We selected a sample of patients aged 3 months to 15 years from the catchment population corresponding to Health District II of the city of Zaragoza, Spain, which includes 45379 children aged 0–14 years.
For the purpose of patient selection, we chose the enzyme alanine aminotransferase (ALT, previously GPT) due to its higher specificity for abnormal liver function, including every child with serum levels exceeding predefined thresholds for sex and age. We established the reference values based on those proposed by Lamireau et al.18 in 2014, which in turn took into account the studies conducted by England et al.10 in 2009 and by Schwimmer et al.11 in 2010, defining the upper limits of normal (ULNs) for 3 age groups:
- •
3 months to 18 months: 60IU/L in boys and 55IU/L in girls.
- •
18 months to 12 years: 40IU/L in boys and 35IU/L in girls.
- •
12 years to 15 years: 26IU/L in boys and 22IU/L in girls.
We included all patients in the catchment population of the selected health district with elevation of ALT past the established thresholds with the test ordered for any reason at the hospital or in primary care levels in a 6-month period (January 1–June 30, 2016). We excluded all patients with a pre-existing disease or treatment plan that justified serum elevation of ALT and patients with elevation of ALT detected prior to the period under study. Thus, the final sample included all patients in the catchment area of the paediatric services of a health district with hypertransaminasaemia in the absence of apparent liver disease. We made a separate evaluation and analysis of patients in whom an aetiological diagnosis was made with the tests or levels requested in the first blood test order.
We were able to select patients through the evaluation of all blood tests performed by the Department of Clinical Biochemistry of the Hospital Universitario Miguel Servet in Zaragoza (Spain), which is tasked with performing all blood tests ordered in the health district. The levels of ALT were measured with a Beckman AU5800 clinical chemistry analyser (pyridoxal-5-phosphate method).
For the selected patients, we collected information on every blood test performed thereafter and analysed by any of the biochemistry departments of the health system of Aragon by reviewing the electronic health records, with the main goal of assessing the persistence of hypertransaminasaemia, in addition to assessing other variables such as cytolysis, cholestasis, liver function and investigation of potential aetiologies.
We defined adequate follow-up as performance of additional blood tests until abnormal levels normalised, or the underlying cause was identified. We defined inadequate follow-up as lack of follow-up testing in the 12 months that followed the initial finding. Clinical practice guidelines even recommend an additional follow-up test after the levels normalise in patients with unexplained hypertransaminasaemia, as the levels could fluctuate.3,4 In our study, we did not consider absence of a new test after resolution of elevation inadequate follow-up.
We performed the statistical analysis with the software SPSS version 20.0, using the chi square test, Student t test and analysis of variance and defining statistical significance as a p-value of less than 0.05.
The study was approved by the Clinical Research Ethics Committee of Aragon (CEICA).
ResultsWe analysed 572 panels with elevation of ALT serum levels corresponding to 403 patients aged 3 months to 15 years and performed in the period under study. They amounted to 8.4% of all ALT tests and 2.9% of all tests analysed by the Department of Biochemistry during the same time period. We excluded 83 patients that had a pre-existing condition or receiving treatment that could account for the elevation (chemotherapy, neuromuscular disease, chronic liver disease, immediate postoperative period, paracetamol poisoning, etc.) and 15 asymptomatic patients undergoing evaluation for hypertransaminasaemia detected before the study period. Thus, in 305 patients there was no apparent cause of hypertransaminasaemia. In 69 of them a probable aetiological diagnosis could be made based on the results of the blood tests of additional diagnostic tests performed at the same time (infectious mononucleosis in 62.3%, post-infectious acute myositis in 20.3%, coeliac disease in 2.9%, etc.).
Clinical guidelines recommend a follow-up test in these patients once the episode of intercurrent disease has resolved to confirm normalisation of the levels, as the identified disease may be masking an underlying condition. In our sample, we found that in the group of patients given a diagnosis of acute disease that could explain the aetiology, normalisation of the enzyme levels was only observed following resolution of the disease in 33.3%.
Thus, we identified 236 patients without elevation of ALT before the period under study and without an apparent health condition or therapeutic intervention prior to or at the time of testing that could justify it. The analysis included this subset of patients.
Of these patients, 47.5% were female (112 patients), and the age distribution was 3.4% aged 3–18 months (8 patients), 58.5% aged 18 months to 12 years (138 patients) and 38.1% aged 12–15 years (90 patients). The tests were ordered at the level of primary care level in 55.9% of cases (132 patients), hospital-based outpatient care in 33.1% (78 patients), paediatric emergency care in 6.8% (16 patients) and inpatient care in 4.2% (10 patients).
The initial level of ALT was less than twice the established ULN in 75.8% (179 patients), between twice and 5 times the ULN in 20.3% (48 patients) and greater than 5 times the ULN in 3.8% (9 patients).
The reason/complaint that led to ordering the initial blood panel was digestive/gastrointestinal in 27.5% (65 patients), acute infection in 16.9% (40 patients), or routine testing that led to an incidental finding in 9.7% (23 patients), preoperative testing with incidental finding in 5.9% (14 patients) and other in 39.8% (94 patients).
In our sample, 24.57% of the patients (58 patients) were in treatment with a drug prescribed by the paediatrician in the days that preceded the collection of the first blood sample. The most frequent drugs being used were anti-infective drugs, chiefly oral amoxicillin (18 patients), oral amoxicillin-clavulanic acid (12 patients) and oral penicillin (5 patients). We were unable to obtain data on over-the-counter medication (paracetamol, NSAIDs, cough medicine, homoeopathic remedies, etc.).
In addition to liver function tests, other measurements or diagnostic tests were ordered for investigation of the aetiology of hypertransaminasaemia, such as thyroid function tests (48.3%, 114 patients), markers of coeliac disease (27.1%, 64 patients), serum immunoglobulins (22.5%, 53 patients), serological tests (17.8%, 42 patients), abdominal ultrasound (11%, 26 patients), autoantibodies (6.4%, 15 patients), ceruloplasmin (4.2%, 10 patients), serum copper (3.8%, 9 patients), alfa-1 antitrypsin (3%, 7 patients), total serum protein (2.5%, 6 patients), reducing substances in urine (0.8%, 2 patients), copper in urine (0.4%, 1 patient) or Doppler ultrasound (0.4%, 1 patient).
Of these patients, 34.3% underwent follow-up testing (81 patients), which revealed persistence of abnormal levels in 46.8% (37 patients). However, in 66.5% (157 patients) testing was not performed again in the 12 months that followed the abnormal result.
When it came to the time elapsed until laboratory tests were performed for follow-up in patients for who it was requested, it was of 30 days of less in 40.7% (33 patients) and greater than 30 days in 59.2% (48 patients), with a mean of 70.6 days (median, 46 days). The time elapsed to follow-up testing was significantly shorter in patients with ALT levels greater than twice the ULN (79.9+66.2 days) compared to patients with levels less than twice the ULN (55.7+63.9 days) and also in inpatients (75.03+77.3 days) compared to outpatients (66.7+60.3 days) (P<.05).
Overall, the follow-up after detection of unexplained hypertransaminasaemia was not adequate in 70.8% of cases (167 patients). Thus, in our sample, adequate follow-up was only performed in 29.2% de los patients (Fig. 1).
When we analysed the variables that could explain the adequacy or inadequacy of the follow-up, we did not find an association between this variable and age, sex, the source of the order or the history of pharmacological treatment, but we did find an association with the initial degree of elevation of ALT (Table 1).
Variables under study in the sample at the time of initial blood testing, overall and in the adequate and inadequate follow-up groups.
| Inadequate follow-up | Adequate follow-up | Total | P | |
|---|---|---|---|---|
| Age in years (mean±SD) | 9.48±4.75 | 8.16±4.95 | 9.9±4.84 | .057 |
| Use of medication (%) | 21.6% | 31.9% | 24.6% | .099 |
| Female (%) | 48.5% | 44.9% | 47.5% | .361 |
| ALT<2×ULN (%) | 80.8% | 63.8% | 75.8% (of total sample) | .01 |
| ALT>2×ULN (%) | 18% | 26.1% | 20.3% (of total sample) | |
| ALT>5×ULN (%) | 1.2% | 10.1% | 3.8% (of total sample) | |
| Settinga (PC/HBC) | 74.2%/66.3% | 25.8%/33.7% | 55.9%/44.1% | .197 |
HBC, hospital-based care (outpatient care, emergency care and inpatient care); PC, primary care; SD, standard deviation; ULN, upper limit of normal.
In the group of patients with adequate follow-up, 13% (9 patients) received a diagnosis that explained the elevation of serum transaminases. Four received a diagnosis of non-alcoholic fatty liver disease, 2 of autoimmune hepatitis, 1 of hepatorenal polycystic disease, 1 of liver haemangioma and 1 of hepatic focal nodular hyperplasia. On the other hand, when it came to patients with persistent transaminase elevation, in 2 it was not possible to determine the cause of hypertransaminasaemia during the follow-up despite an exhaustive evaluation (including a normal liver biopsy) (Table 2).
Patients with a diagnosis that explained hypertransaminasaemia or unexplained hypertransaminasaemia despite a full aetiological investigation.
| Diagnosis | Age | Initial ALT elevation (× ULN) | Reason for ordering | Setting |
|---|---|---|---|---|
| Non-alcoholic fatty liver disease | 14 years | <2 | Hypopituitarism follow-up | Endocrinology clinic |
| Non-alcoholic fatty liver disease | 9 years | 2–5 | Thalassemia follow-up | Primary care |
| Non-alcoholic fatty liver disease | 13 years | <2 | Nonspecific obesity | Endocrinology clinic |
| Non-alcoholic fatty liver disease | 11 years | <2 | Abdominal pain | Emergency department |
| Autoimmune hepatitis | 2 years | 2–5 | Suspected coeliac disease | Gastrointestinal clinic |
| Autoimmune hepatitis | 20 months | <2 | Vomiting | Primary care |
| Hepatic haemangioma | 13 years | <2 | Joint pain | Rheumatology clinic |
| Hepatorenal polycystic disease | 20 months | 2–5 | Weight faltering | Primary care |
| Hepatic focal nodular hyperplasia | 11 years | <2 | Abdominal pain | Primary care |
| Unexplained hypertransaminasaemia | 6 months | 2–5 | Follow-up of operated omphalocele | Neonatal care |
| Unexplained hypertransaminasaemia | 20 months | >5 | Prolonged diarrhoea | Primary care |
Two thirds of the patients that received a final diagnosis of liver disease had initial levels of ALT less than twice the established ULN, and we did not find initial levels greater than 5 times the ULN in any of the patients.
DiscussionSerum elevation of liver enzymes is an increasingly frequent incidental finding in blood tests ordered in asymptomatic patients or in the context of mild symptoms in the paediatric population.3,4 This elevation is a nonspecific feature found in numerous conditions, but it may also be the initial clinical manifestation of potentially serious progressive liver disease. Therefore, it is important to monitor these levels to confirm normalisation or to carry out a broader evaluation to establish the aetiology of the elevation. The aim of our study was to analyse the follow-up in current clinical practice of paediatric patients with hypertransaminasaemia in our region. To do so, we selected a 6-month period back enough in time to have allowed a comprehensive investigation of hypertransaminasaemia to be completed.
Normal serum liver enzyme levels have not been specifically established in the paediatric population. The thresholds established in studies on the subject vary widely, although most propose adjustments based on sex and age among other possible factors (ethnicity, country of origin, laboratory etc.).7–27 Thus, we believe it is necessary to establish universal thresholds to define the need for follow-up or diagnostic evaluation adjusted for different variables, such as age and sex.
In our study we used the thresholds established by Lamireau et al.,18 who took into account the studies previously conducted by England et al.10 and Schwimmer et al.11 We established an age range for the sample of 3 months to 15 years, excluding the neonatal period and concluding at the age limit used to define eligibility for paediatric services in the hospital where the study was conducted. We divided the sample into 3 age groups based on the criteria applied in the cited studies: 3–18 months, 18 months to 12 years and 12–15 years.
In our sample, we found a slight predominance of the male sex, which was consistent with other studies in the literature.10,13,28–30
Among the multiple reasons for ordering liver function tests, the most frequent overall are acute infectious diseases, as reported in international publications,29,30 especially in patients with acute pharyngotonsillitis. As for the setting from which testing is ordered, we ought to highlight the role of primary care in its interpretation and initial follow-up, most frequently of a chance finding in otherwise healthy patients with nonspecific symptoms or during a routine checkup in asymptomatic patients.1,29,30
The times that must elapse between follow-up laboratory tests vary depending on the consulted guidelines.3,4,31 In our sample, the mean time elapsed to the first follow-up test was 70.6 days, and this time was significantly shorter in patients with severe ALT elevation and in inpatients compared to outpatients, probably because more blood tests are performed overall in hospitalised patients.
We found that 24.5% of patients received some form of pharmacological treatment in the days preceding the initial blood test, usually with an anti-infective agent (chiefly oral amoxicillin, oral amoxicillin-clavulanic acid or oral penicillin), which was consistent with the previous literature.3,32,33 One of the limitations of our study was that we could not obtain exhaustive data on the drugs used by patients (such as paracetamol, ibuprofen, mucolytic agents, etc., which can be purchased without a prescription) or their dosage and duration of treatment, which would not be documented in the health records of the patient, or the use of natural or nonpharmacological treatments potentially associated with transaminase elevation.
In our study, adequate follow-up of hypertransaminasaemia was performed in less than one third of the patients, and a liver disease that explained the abnormal findings was identified in 13% of them. It is possible that some of the patients underwent follow-up testing in private health care facilities or in a different autonomous community in Spain, although it is fair to assume that if initial testing was performed in a public health care facility in Aragon, the follow-up would also be performed in the public health care system of this autonomous community.
In our sample, we did not find an association between adequate follow-up and age, sex, setting where testing was ordered or the history of pharmacological treatment, but we found a greater frequency of follow-up in patients with higher initial elevations of ALT, which suggests that there is a tendency to underestimate the significance of mild elevation of ALT as a potential marker of liver disease.
The obvious concern raised by these findings is the possible presence of liver disease in paediatric patients that were not followed up due to the absence or nonspecificity of their clinical manifestations and that may present later with progressive liver involvement, in whom an opportunity of diagnosis has been lost.
The low frequency of follow-up in our sample could be due to the lack of agreement between different guidelines as regards follow-up of transaminase elevation in the paediatric population,30,18,28,35–38 in addition to the lack of consensus on the cut-off points used to interpret transaminase levels or the potential misconception in health care professionals that liver disease can be ruled out in patients with mild liver enzyme elevation, which is clearly refuted by the findings of our study.
Other case series in the adult population have found higher proportions of adequate follow-up. For example, a study published in 201934 that analysed the follow-up of hypertransaminasaemia in a sample of adults found that liver function tests had been repeated within a year in 68% of patients with abnormal results and within 2 years in 80%, and that follow-up testing was not performed following the initial test results in only 11.7% of these patients. It is also important to consider that in the adult population there are additional factors that could give rise to hypertransaminasaemia in the absence of disease, mainly alcohol consumption, which is not always disclosed by patients,35,36 and which, with exceptions in the adolescent population, is not at play in the paediatric population.
Our study demonstrates that liver disease does not always manifest with substantial transaminase elevation, as two thirds of the patients in our sample in who a liver disease was identified had elevated ALT levels less than twice the ULN. In this regard, efforts to improve care should focus on ensuring adequate follow-up of asymptomatic patients with mild transaminase elevation.
Although many clinical practice guidelines have been published on the subject for the adult population, and several for the paediatric population,3,18–20,35–38 the care protocols applied in children and/or adolescents vary widely in the applied cut-off points and the approaches to management. Our intent in conducting this study was to underscore the importance of follow-up in every single child with hypertransaminasaemia, independently of age, the degree of liver enzyme elevation or the exposure to pharmacological treatment, ideally following a standardised care protocol. Needless to say, health care professionals must be made aware of the importance of such follow-up.
In conclusion, in our health care area and in the period under study, the follow-up of most children with elevation of ALT in the absence of apparent liver disease was inadequate, especially in those with mild elevation. This constitutes a missed opportunity for the early diagnosis and treatment of potential liver disease in a large number of children. All children with unexplained hypertransaminasaemia should undergo evaluation with application of consensus-based thresholds and with a standardised approach.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández Ventureira V, Ros Arnal I, Rodríguez Martínez G, García Rodríguez B, García Romero R, Ubalde Sainz E. Evaluación del seguimiento de niños con hallazgo de hipertransaminasemia. An Pediatr (Barc). 2021;94:359–365.