Understanding the variations of abdominal vascular structures is important for preventing complications of abdominal surgical procedures for gastrointestinal disease such as necrotizing enterocolitis or others that may arise in patients with congenital cardiac disease. We analysed the coeliac trunk and its branches in children with congenital heart disease to determine whether there is a greater prevalence of associated vascular abnormalities.
MethodsWe retrospectively analysed thoracic computed tomography (CT) angiograms performed in our hospital in paediatric patients with congenital heart disease. We documented the anatomical variations observed in abdominal sections in which the coeliac trunk and hepatic arteries were included in the field of view. We used the Uflacker classification to describe anatomical variants of the coeliac trunk, and the Michels classification and its modified version (Hiatt classification) to describe the anatomy of the hepatic artery system.
ResultsOur study included 178 patients with congenital heart disease. We identified coeliac trunk variants in 10.7% of the patients. Gastrosplenic trunk was to the most prevalent variant, amounting to 5.6% of total cases. We found hepatic artery variations in 19.1% of the patients. According to the Michels classification, the prevalence of accessory left hepatic artery arising from the left gastric artery as 4.5%, compared to 6.7% based on the Hiatt classification.
ConclusionThe prevalence of coeliac trunk and hepatic artery variations in patients with congenital heart disease was not greater in our study compared to other series in the literature. Clinicians must be vigilant about the variations detected in multislice CT scans to avoid complications resulting from vascular abnormalities, especially in patients who undergo abdominal surgery.
Comprender las variaciones de las estructuras vasculares abdominales es importante para prevenir complicaciones de procedimientos quirúrgicos abdominales por enfermedades gastrointestinales, como la enterocolitis necrotizante u otras que pueden desarrollarse en pacientes con cardiopatías congénitas. Se examinará el tronco celíaco y sus ramas en niños con cardiopatía congénita para determinar si hay una mayor prevalencia de anomalías concomitantes.
MétodosExploración retrospectiva de pacientes pediátricos con cardiopatías congénitas sometidos a una angiotomografía computarizada (angio-TC) en nuestro hospital. Se registraron las variaciones anatómicas en secciones abdominales en las que el plano de adquisición incluía el tronco celíaco y las arterias hepáticas. Se utilizó el método de Uflacker para describir las variantes anatómicas del tronco celíaco. Las variaciones en el sistema de la arteria hepática se caracterizaron mediante la clasificación de Michels y la versión modificada de Hiatt.
ResultadosLa muestra incluyó a 178 pacientes pediátricos con cardiopatías congénitas. Se encontraron variaciones de la arteria celíaca en el 10,7% de los pacientes. La variante más prevalente era el tronco gastroesplénico, que representaba el 5,6% de todos los casos. La prevalencia de variaciones de la arteria hepática fue del 19,1%. La prevalencia observada de arteria hepática izquierda accesoria con origen en la arteria gástrica izquierda fue del 4,5%, según la clasificación de Michels y del 6,7% según la clasificación de Hiatt.
ConclusiónEn nuestro estudio, no se observó una prevalencia mayor de variaciones del tronco celíaco y de la arteria hepática en pacientes con cardiopatías congénitas en comparación con lo descrito en series previas. Los médicos deben prestar atención a las variaciones detectadas en las tomografías computarizadas multicorte para evitar complicaciones derivadas de variaciones vasculares, especialmente en pacientes sometidos a cirugía abdominal.
The arterial blood supply of the gastrointestinal tract is provided by branches of the abdominal aorta at three levels (coeliac trunk and superior and inferior mesenteric arteries).Variations in these vascular systems are caused by abnormalities in vascular embryogenesis at different stages of development.1
Understanding the variations of abdominal vascular structures is important for preventing complications during and after abdominal surgical procedures such as liver transplantation, laparoscopic surgery or tumour removal as well as abdominal interventional radiology procedures.2 It is known that the frequency of iatrogenic vascular damage increases with the presence of abnormal vascular architecture and anatomic variations.3
Congenital heart diseases are a significant cause of morbidity and mortality in children, and their incidence is estimated at 5–9 per 1000 live births.4,5 In these patients, congenital vascular abnormalities and cardiopulmonary bypass may cause gastrointestinal complications.6 Because of the latter, some patients may require abdominal surgery.
Although digital subtraction angiography (DSA) is the gold standard for vascular imaging, multislice computed tomography angiography (MSCTA) is replacing DSA on account of advantages such as obtaining thinner slices, faster image collection, allowing the generation of 3D reconstructed images and being noninvasive. Computed tomography angiography is extremely effective for displaying both normal and abnormal vascular architecture.7,8
Although echocardiography is the first step in the detection of congenital heart disease, thoracic CT angiography is used to investigate echocardiographic findings in greater detail and to assess the effectiveness of treatment in operated patients.9
Although there are numerous studies reporting coeliac trunk and hepatic artery structure and variations, to our knowledge there is no study analysing coeliac artery and hepatic artery variants in paediatric patients with congenital heart disease.10,11 Our aim was to determine the presence of concomitant alterations by evaluating the coeliac trunk and its branches through thorax CT angiography in patients with congenital heart disease.
Material and methodsStudy design and sampleWe conducted a retrospective cross-sectional study by collecting data on paediatric patients with congenital cardiac disease for whom it was possible to evaluate the coeliac trunk and hepatic arteries in the abdominal region investigated using thorax CT data. The inclusion criteria were: paediatric patient, congenital heart disease, having undergone a thoracic CT angiography scan in our hospital and the possibility of evaluating the coeliac trunk and hepatic artery branches in the abdominal sections of the recorded thorax CT angiogram. Of all children who had undergone a thorax CT angiography scan, we excluded those without congenital heart disease and those whose abdominal arteries could not be visualized in the thorax CT angiogram.
CT angiography techniqueComputed tomography angiography scans of the thorax were conducted with a multislice CT scanner (Philips Ingenuity Core 64 slice scanner). We maintained the standard 80 kVp dose in the settings, and the mAs were set automatically by the device based on the weight of the patient. During the scan, 1.5−2 mL/kg of non-ionic contrast agent was administered through an automated injector system via peripheral vascular access (e.g., antecubital fossa, forearm, back of the hand).
Analysis of the vascular system and classification of vascular variationsImages taken with a slice thickness of 1–3 mm in the axial plane were reconstructed with a section thickness of 0.625 mm, and MPR (multi planar reformation), MIP (maximum intensity projection), three-dimensional volume rendering (3D VR) images were obtained in the coronal and sagittal planes. The imaging protocol did not include any additional image acquisition for assessment of abdominal vascular structures. The images were analysed using the picture archive and communication system (PACS). In the abdominal portions included in the sections, we assessed the anatomy and variations of the coeliac trunk and hepatic artery. Two radiologists interpreted the scans independently, documenting any anatomic variations. If there was disagreement between their interpretations, they were resolved by consensus.
We applied the Uflacker classification to characterise the anatomical variants of the coeliac trunk. For the variations of the hepatic artery system, we applied the Michels classification, an internationally approved classification scheme developed in 1966, and the Hiatt classification, a modification of the Michels system proposed in 1994.
The study was approved by the ethics committee of our hospital (Dr. Sami Ulus Paediatrics Hospital). The study received a waiver of informed consent.
Statistical analysisWe have expressed continuous variables, except those that were not normally distributed like age, as mean and standard deviation. We used the Kolmogorov-Smirnov test to assess normality. The imaging findings and variation types were expressed as percentages. The analysis was carried out with the software IBM SPSS version 22.0.
ResultsA total of 305 patients with various pre-existing diagnoses underwent a CT angiography scan of the thorax at our hospital between January 2017 and July 2022 before the study. We excluded 111 patients because they did not have congenital heart disease and 16 because the coeliac trunk and hepatic artery were not within the field of view. The final study sample comprised 178 patients, 64% (n = 114) male and 36% (n = 64) female. The age range of the sample was 1 day to 224 months and the median was 85 months. Table 1 shows the distribution of the congenital heart diseases that were the indication for the CT angiogram of the thorax.
Indications for CT angiography of the thorax in patients with congenital heart disease.
| Congenital heart disease | n (%) |
|---|---|
| Coarctation of the aorta | 68 (38.2%) |
| Transposition of the great arteries | 36 (20.2%) |
| Tetralogy of Fallot | 17 (9.5%) |
| Mitral valve prolapse | 13 (7.3%) |
| Bicuspid aortic valve | 11 (6.1%) |
| Interrupted aortic arch | 10 (5.6%) |
| Anomalous pulmonary venous return | 9 (5.4%) |
| Pulmonary stenosis | 5 (2.8%) |
| Tricuspid atresia | 4 (2.2%) |
| Heterotaxy syndrome | 3 (1.6%) |
| Ebstein anomaly | 2 (1.1%) |
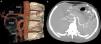
Based on the the Uflacker classification, 159 patients (89.3%) had normal coeliac trunk anatomy (type I) (Fig. 1), 10 cases (5.6%) had gastrosplenic trunk (type V) (Fig. 2), 4 cases (2.2%) coeliac-mesenteric trunk (type VI), 3 (1.7%) absence of the coeliac trunk (type VIII), and 2 cases (1.1%) hepatosplenic trunk (type II) (Table 2).
Female patient aged 16 years with pulmonary artery agenesis.
Gastrosplenic trunk visualized in 3D VR (a) and axial plane MIP images (b), with the common hepatic artery originating from the superior mesenteric artery. CHA, Common hepatic artery; GDA, gastroduodenal artery; LGA, left gastric artery; SA, splenic artery; SMA, superior mesenteric artery.
Coeliac trunk variations according to Uflacker classification.
| Type | Description | n (%) |
|---|---|---|
| I | Classic coeliac trunk | 159 (89.3%) |
| II | Hepatosplenic trunk | 2 (1.1%) |
| III | Hepatogastric trunk | 0 (0%) |
| IV | Hepatosplenicmesenteric trunk | 0 (0%) |
| V | Gastrosplenic trunk | 10 (5.6%) |
| VI | Coeliac-mesenteric trunk | 4 (2.2%) |
| VII | Coeliac-colic trunk | 0 (0%) |
| VIII | Absence of coeliac trunk | 3 (1.8%) |
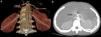
According to the Michels classification, 80.9% of the patients had normal (type 1) hepatic artery anatomy. The most prevalent variation (4.5%) was an accessory left hepatic artery (type 5) originating from the left gastric artery (LGA) (Fig. 3). In addition, the anatomy in 9 patients could not be defined using the Michels classification. In 5 cases, the variation consisted of the main hepatic artery originating from the abdominal aorta, in 2 of the right hepatic artery originating from the abdominal aorta, in 1 of the right hepatic artery originating from the coeliac trunk, and in 1 of the connection of the main hepatic artery originating from the abdominal aorta and the right hepatic artery originating from the superior mesenteric artery (SMA) (Fig. 4).
Female patient aged 15 years with scimitar syndrome.
3D VR (a) and MIP (b) images show the accessory left hepatic artery branching from the left gastric artery. aLHA, accessory left hepatic artery; CHA, Common hepatic artery; GDA, gastroduodenal artery; LHA, left hepatic artery; LGA, left gastric artery; SA, splenic artery; SMA, superior mesenteric artery.
According to the Hiatt classification, 80.9% of patients had normal (type 1) hepatic artery anatomy. The most prevalent variation, at 6.7%, was replaced or accessory left hepatic artery (type 2) arising from the LGA. There were also 4 patients with anatomy that had not been classified in the previous literature. They included 2 patients with right hepatic arteries arising from the abdominal aorta, 1 with right hepatic artery arising from the coeliac trunk, and 1 with combined common hepatic artery arising from the abdominal aorta and right hepatic artery arising from in the SMA.
DiscussionMultislice CT angiography is used to diagnose congenital heart diseases and to assess treatment response in the postoperative period.12 High-resolution MPR, MIP, and VR imaging derived from thin-section scans contribute significantly to the study of vascular anatomy and abnormalities.13
To date, no research has been conducted on the incidence of vascular abnormalities in patients with congenital heart disease. However, abnormalities in the vascular structure of the heart in the context of congenital heart disease suggest that it may be associated with numerous vascular anomalies in abdominal arterial structures. According to Güney et al., the incidence of gastrointestinal complications is significant, particularly in patients with cyanotic congenital heart diseases or operated for congenital heart disease.6 The most common complication is necrotising enterocolitis, with a lower incidence of liver laceration. These complications frequently require abdominal surgery.
Understanding the structural differences of the coeliac trunk and the hepatic artery is critical for preventing complications before and after surgery, particularly in the case of liver transplantation or abdominal surgical or interventional radiology procedures.1,14
Only half of the population has the classic anatomy of the coeliac trunk and hepatic artery. The coeliac trunk arises from the aorta as the first branch of the abdominal aorta and gives rise to branches such as the common hepatic artery, splenic artery, and left gastric artery in this architecture. The common hepatic artery is the thickest branch in foetuses and infants. The common hepatic artery branches into the proper hepatic artery and the gastroduodenal artery at the level of the first part of the duodenum. At the level of the porta hepatis, the proper hepatic artery branches into the right hepatic artery, middle hepatic artery, and left hepatic artery.15,16
There are many studies in the literature regarding coeliac trunk and hepatic artery variations based on findings from cadaver dissections, multislice CT and DSA. Haller described the classic branching of the coeliac trunk for the first time in 1756.16,17 Various articles report a prevalence of normal anatomy ranging from 72% to 89%. The main studies on coeliac trunk and hepatic artery variations in adults were conducted by Michels,11 Hiatt,3 Song15 and Lezzi et al.17 Caliskan et al. conducted a retrospective study in 174 children who underwent multidetector CT angiography due to trauma or liver transplantation. The authors found normal anatomy in 90.2% and coeliac trunk variations in 9.8%. Hepatosplenic trunk was the most frequently identified coeliac trunk variant. When it came to the hepatic artery, there was normal anatomy in 64.4% of cases and variations in 35.6%.The most prevalent variations were replaced right hepatic artery arising from the SMA and replaced left hepatic artery arising from the LGA.18
In our study, the anatomy of the coeliac trunk was normal in 89.3% of the cases and variations were found in 10.7%. Gastrosplenic trunk (5.6%) was the most prevalent variant. There were no cases of hepatogastric trunk, hepatosplenomesenteric trunk or coeliac-colic trunk. The frequency of normal anatomy in our study was comparable to the frequency reported in previous studies.16,19 While gastrosplenic trunk was the most common coeliac trunk variation in our sample, hepatosplenic trunk was more prevalent in other studies.
The Michels autopsy study established ten types of hepatic artery anatomy, and in a study of 200 patients, the prevalence of normal anatomy was 55% and the prevalence of variant anatomy was 45%. The most common variant anatomy was defined as replaced right hepatic artery (type III) originating from the SMA.11Table 3 compares the prevalence of hepatic artery variations in our study to the prevalence in the study by Michels.
Prevalence of hepatic artery variations compared to the Michels study, n (%).
| Type | Michels study (n = 200) | Present study (n = 178) |
|---|---|---|
| I | 110 (55%) | 144 (80.9%) |
| II | 20 (10%) | 4 (2.2%) |
| III | 22 (11%) | 3 (1.7%) |
| IV | 2 (1%) | 0 (0%) |
| V | 16 (8%) | 8 (4.5%) |
| VI | 14 (7%) | 1 (0.6%) |
| VII | 2(1%) | 2 (1.1%) |
| VIII | 4 (2%) | 0 (0%) |
| IX | 9 (4.5%) | 7 (3.9%) |
| X | 1 (0.5%) | 0 (0%) |
| Other | 0 (0%) | 9 (5.1%) |
In a study of the surgical anatomy of the hepatic artery in 1000 individuals based on operative notes conducted in 1994, Hiatt et al. found normal anatomy in 75.7% of patients. With a prevalence of 10.6%, the most common variation was replaced or accessory right hepatic artery (type 3).3 In our study, we found normal hepatic artery anatomy in 80.9% of cases and a prevalence of variation of 19.1%. Accessory left hepatic artery arising from the LGA was the most prevalent variant, amounting to 4.5% of the total cases (Table 4).
ConclusionIn conclusion, the detection of any variations in the abdominal arteries is crucial, as patients with congenital heart diseases may require abdominal surgery. The incidence of coeliac trunk and hepatic artery variations in patients with congenital heart disease found in our study was similar to the incidence reported in the reviewed literature. However, due to the low frequency of congenital heart diseases, the number of cases in our study was small, and studies in larger samples should be conducted to contribute to the literature.
FundingThe study received no funding.
Conflict of interestsAuthors are required to disclose financial or non-financial interests that are directly or indirectly related to the work submitted for publication.