Around 2000 children and adolescents die each year in Spain, however, we know little about the particularities of deaths in paediatrics. The purpose of this study is to document the characteristics of patients who die in the care of paediatric palliative care teams in Spain.
Patients and MethodsRetrospective, descriptive, multicentre study. Fourteen teams from all over the country participated.
ResultsData were obtained from 164 patients. In most cases the underlying disease stemmed from oncological, neurological or neuromuscular processes. The median age at death was 6.9 years (RIC 11.2). The median follow-up time by the team was 0.3 years (RIC 0.8 years). The most frequent symptoms in the last week of life were dyspnoea, pain, increased secretions and sleep disorders. The median number of drugs administered to each patient one week prior to death was 6 (RIC 4). The place of death for 95 of the patients (57.9%) was hospital while 67 (40.9%) died at home.
ConclusionsThere was a wide age range of patients and they had substantial exposure to polypharmacy. The follow-up time shows that patients have late access to palliative care programmes. An effort should be made to introduce this care earlier rather than relegating it to the end of life. In Spain there is an unequal distribution of resources and not all teams can provide care at home. The place of death should be interpreted with caution.
Cada año fallecen en España alrededor de 2000 niños y adolescentes; sin embargo, conocemos poco las particularidades que envuelven a la muerte en pediatría. El objetivo de este estudio es documentar las características de los pacientes que fallecen a cargo de los Equipos de Cuidados Paliativos pediátricos en España.
Pacientes y MétodosEstudio retrospectivo, descriptivo y multicéntrico. Participaron 14 equipos de todo el territorio nacional.
ResultadosSe obtuvieron datos de 164 pacientes. En la mayoría la enfermedad de base eran procesos oncológicos, neurológicos y neuromusculares. La mediana de edad al fallecimiento fue de 6.9 años (RIC 11.2). La mediana de tiempo de seguimiento por el equipo fue de 0.3 años (RIC 0.8 años). Los síntomas más frecuentes en la última semana de vida fueron disnea, dolor, aumento de secreciones y trastornos del sueño. El número de fármacos que se administraban a cada paciente una semana previa al fallecimiento tuvo una mediana de 6 (RIC 4). El lugar de fallecimiento de 95 de los pacientes (57.9%) fue el hospital y de 67 (40.9%) fue su domicilio.
ConclusionesLos pacientes presentaban un amplio rango de edad y una exposición sustancial a la polifarmacia. El tiempo de seguimiento nos muestra el acceso tardío a los programas de Cuidados Paliativos, deberíamos hacer un esfuerzo para la introducción temprana de estos cuidados y que no quede relegada al final de vida. En España existe una distribución desigual de recursos, sin que todos los equipos tengan la posibilidad de atención domiciliaria, por lo que el lugar de fallecimiento debemos interpretarlo con cautela.
Every year, approximately 2000 children and adolescents in Spain die,1 and 15 000 live with a life-threatening disease.2
In recent decades, thanks to the increasing sophistication and technological advances of medical care, there has been a decrease in child and adolescent mortality in our country, but death continues to occur in paediatrics, upsetting the natural order of life. The causes of death include conditions that develop abruptly and unexpectedly in a previously healthy patient and life-threatening or life-limiting diseases that impair quality of life.2 In the latter, the most frequent conditions are diseases that develop in the perinatal period, congenital malformations, chromosomal abnormalities and malignant tumours.3
When a minor receives a diagnosis of life-limiting or life-threatening disease, care is usually provided by a broad range of professionals in different fields, possibly including specialists in paediatric palliative care (PPC). The aim of PPC is to improve the quality of life of patients and families through an active and dynamic approach to care from the moment of diagnosis to death and bereavement.4,5
Palliative care is one of the pillars of care at the end of life in the paediatric population,6 although its delivery is not always guaranteed, with considerable variability in the access to these services throughout the world.7
In Spain, the first PPC team (PPCT) was instituted in 1991 in the Hospital Sant Joan de Deu. Slowly, other hospitals started to establish their own teams, with the most rapid growth at the national level taking place in the past 10 years. To date, no study has been conducted to analyse the profile, demand and delivery of these services in patients who die in the care of these teams.
The aim of our study was to document the demographic and clinical characteristics and treatment received by patients who died in the care of specialised PPCTs in Spain to improve our understanding of the particularities of death in the paediatric population and contribute reference data for future studies.
Patients and methodsWe conducted a multicentre retrospective descriptive study between January 1 and December 31, 2019. A total of 14 PPCTs throughout Spain participated in data collection (Table 1).
Specific paediatric palliative care teams (PPCTs) that participated, and number of patients included per team.
| Hospital | Number of patients managed by PPCT in 2019 N | Number of patients that died in the care of the PPCT in 2019 N | Number of patients included in study n (%) |
|---|---|---|---|
| Hospital San Juan de Dios de Barcelona | 321 | 59 | 42 (25.6%) |
| Hospital Universitario Infantil Niño Jesús de Madrid | 127 | 40 | 40 (24.4%) |
| Hospital Regional Universitario de Málaga | 59 | 17 | 17 (10.4%) |
| Hospital Universitario Virgen del Rocío de Sevilla | 63 | 16 | 16 (9.8%) |
| Hospital Universitario Son Espases de Mallorca | 79 | 9 | 9 (5.5%) |
| Hospital Universitario Virgen de las Nieves de Granada | 50 | 8 | 8 (4.9%) |
| Hospital Universitario de Cruces de Bilbao | 52 | 6 | 6 (3.7%) |
| Hospital Universitario Miguel Servet de Zaragoza | 87 | 6 | 6 (3.7%) |
| Hospital Clínico Universitario Virgen de la Arrixaca de Murcia | 35 | 5 | 5 (3.0%) |
| Hospital Universitario Torrecárdenas de Almería | 34 | 5 | 5 (3.0%) |
| Hospital Universitario Parc Taulí de Sabadell | 50 | 3 | 3 (1.8%) |
| Complejo Virgen de la Salud de Toledo | 58 | 7 | 3 (1.8%) |
| Hospital General Universitario de Alicante | 96 | 3 | 3 (1.8%) |
| Hospital Universitario Nuestra Señora de Candelaria de Tenerife | 35 | 1 | 1 (0.6%) |
We included every patient managed by the PPCTs during the study period that died in their care, and age was not an exclusion criterion. Data were collected through the review of health records, and we considered patients that died in the care of the team for whom we did not obtain data lost to follow-up.
We collected data on demographic variables such as sex, age, date of diagnosis, date of inclusion in the PPCT caseload and date of death. As regards clinical data, we collected the underlying disease and the symptoms experienced within 7 days of death as well as the drugs and devices used in the week preceding death. We also collected data on variables related to the end of life, such as the cause of death, place of death and withdrawal or withholding of treatment.
To describe the underlying disease, we used the paediatric complex chronic conditions classification system, version 2, developed by Feudtner.8
We conducted an exploratory descriptive analysis of the data, calculating measures of central tendency, dispersion and frequency. We assessed the normality of the distribution by means of the Kolmogorov-Smirnov test as well as analyses of asymmetry and skewness. We summarised quantitative data as mean and standard deviation if they followed a normal distribution, and otherwise as median and interquartile range (IQR). We performed a bivariate analysis using the chi square test or the Fisher exact test as applicable, and analysis of variance (ANOVA) to compare subgroups based on the Feudtner classification, using the Brown-Forsythe test to ensure robustness in case the assumption of homogeneity of variance was not held (which was assessed by means of the Levene test).
ResultsThe sample included a total of 164 patients, 84 female (51.2%) and 80 male (48.8%).
Table 2 presents the distribution by underlying disease, evincing that most of the sample had malignant, neurologic or neuromuscular diseases. We applied the classification of the Association for Children with Life-Threatening or Terminal Conditions and Their Families (ACT)9 considering the underlying disease at diagnosis (Table 3).
ACT group at diagnosis.
| Grupo ACT | n (%) |
|---|---|
| ACT I: Life-threatening conditions for which curative treatment is feasible but may fail | 52 (31.7%) |
| ACT II: Conditions where premature death is inevitable but where there may be long periods of intensive treatment to prolong life | 3 (1.8%) |
| ACT 3: Progressive conditions without curative treatment options where treatment is exclusively palliative and may extend over many years | 69 (42.1%) |
| ACT 4: Presence of neurologic abnormalities that increase the susceptibility to health complications, irreversible but non-progressive conditions that increase the likelihood of premature death | 40 (24.4%) |
The median age at diagnosis was 1.5 years (IQR, 7.6), and the median age at admission to PPC was 5.8 years (IQR, 31.8). We did not find significant differences in the age at diagnosis based on the underlying disease (F3,158 = 1.77; P = .155) or the age at admission to PPC based on the underlying disease (F3,158 = 2.01; P = .114).
The median time elapsed from onset to death was 1.6 years (IQR, 4.3) and the median duration of follow-up of the patient and the family by the PPCT 0.3 years (IQR, 0.8 years). We did not find significant differences in the duration of disease between underlying disease groups (F3,158 = 1.22; P = .309) nor the duration of follow-up based on the underlying disease (F3,158 = 0.79; P = .425).
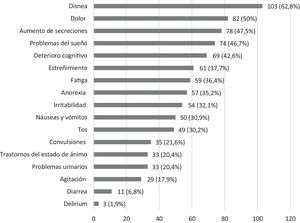
Fig. 1 summarises the symptoms present in the last 7 days of life, with a median of 5.5 symptoms per patient (IQR, 12). The most frequent symptoms were dyspnoea (62.8%), pain (50%), excessive secretions (47.5%) and sleep disorders (46.7%).
We analysed the distribution of symptoms by underlying disease group. We found significant differences in patients with neurologic conditions, who had excessive secretions and seizures more frequently than patients in the rest of the groups, and in the group of patients with malignant disease, with a higher frequency of pain, sleep problems, fatigue, nausea and vomiting, anorexia and mood disorders (Table 4).
Distribution of symptoms by underlying disease group.
| Neurologic (n = 39) n (%) | Malignancy (n = 79) n (%) | Metabolic (n = 15) n (%) | Other (n = 31) n (%) | Χ2 | P | |
|---|---|---|---|---|---|---|
| Dyspnoea | 28 (71.8%) | 47 (59.5%) | 8 (53.3%) | 20 (64.5%) | 2.35 | .506 |
| Pain | 9 (23.1%) | 58 (73.4%) | 8 (53.3%) | 7 (22.6%) | 38.02 | <.001 |
| Excessive secretions | 26 (66.7%) | 26 (32.9%) | 8 (53.3%) | 18 (58.1%) | 14.07 | .003 |
| Sleep problems | 3 (7.7%) | 15 (19%) | 2 (13.3%) | 4 (12.9%) | 13.24 | .039 |
| Cognitive impairment | 15 (38.5%) | 33 (41.8%) | 10 (66.7%) | 11 (35.5%) | 4.48 | .213 |
| Constipation | 11 (28.2%) | 37 (46.8%) | 4 (26.7%) | 9 (29%) | 6.08 | .107 |
| Fatigue | 7 (17.9%) | 44 (55.7%) | 2 (13.3%) | 6 (19.4%) | 25.89 | <.001 |
| Anorexia | 4 (10.3%) | 46 (58.2%) | 3 (20%) | 4 (12.9%) | 37.48 | <.001 |
| Irritability | 9 (23.1%) | 28 (35.4%) | 7 (46.7%) | 10 (32.3%) | 3.22 | .358 |
| Nausea and vomiting | 6 (15.4%) | 35 (44.3%) | 3 (20%) | 6 (19.4%) | 13.90 | .003 |
| Cough | 15 (38.5%) | 21 (26.6%) | 2 (13.3%) | 11 (35.5%) | 4.20 | .240 |
| Seizures | 14 (35.9%) | 9 (11.4%) | 5 (33.3%) | 7 (22.6%) | 10.89 | .012 |
| Mood disorders | 3 (7.7%) | 29 (36.7%) | 0 (0%) | 1 (3.2%) | 26.55 | <.001 |
| Urinary disorders | 7 (17.9%) | 19 (24.1%) | 2 (13.3%) | 5 (16.1%) | 1.61 | .657 |
| Agitation | 3 (7.7%) | 16 (20.3%) | 4 (26.7%) | 6 (19.4%) | 3.92 | .270 |
In the week preceding death, patients were receiving a median of 6 drugs (IQR, 4), and the drugs used most frequently were morphine, used in 90 patients (54.9%), metamizole, used in 75 (45.7%), paracetamol, used in 71 (43.3%), antibiotics, used in 71 (43.3%), midazolam, used in 67 (40.9%), steroids, used in 56 (34.1%), antiepileptics, used in 63 (38.4%), antiemetics, used in 44 (26.8%), fentanyl, used in 33 (20.1%) and gabapentin, used in 33 (20.1%). The most frequent route of administration for morphine was the intravenous route (n = 38; 46.9%), followed by the oral/enteral route (n = 22; 27.1%) and the subcutaneous route (n = 21; 25.9%).
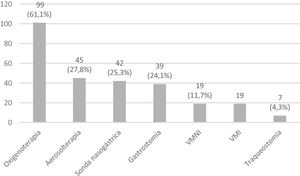
When it came to interventions requiring medical equipment, the most frequently needed intervention was oxygen therapy, used in 101 patients (61.6%) (Fig. 2).
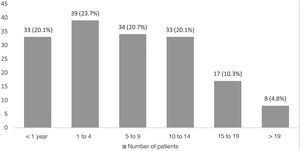
The median age at the time of death was 6.9 years (IQR, 11.2); 65.1% of patients died at ages between 1 and 14 years, 20.1% before age 1 year and 15.1% at ages greater than 14 years (Fig. 3). The cause of death was the progression of the underlying disease in 101 patients (61.6%), a comorbidity in 51 patients (31.1%) and an unexpected cause in only 12 patients (7.3%). In 118 patients (72%) treatment was withheld or withdrawn in agreement with the family. Ninety-five patients (57.9%) died in the hospital and 67 (40.9%) at home, and another 2 patients (1.2%) died during transport from home to the hospital.
DiscussionOur study describes the characteristics of children and adolescents that died during follow-up by a PPCT in 14 Spanish hospitals. The sample represented a broad age range and a wide spectrum of underlying medical conditions, with a substantial prevalence of polypharmacy and technology dependence.
Five salient findings merit being highlighted and discussed. First, when it came to age, we found that some age groups were underrepresented in the sample, in which only one fifth of the patients were younger than 1 year. Given that half of the total paediatric deaths occur in the first year of life, this could suggest underutilization of PPC in this segment of the population. Future analytical studies would be required to determine whether this is due to deficient accessibility, professional practice patterns, organizational factors or low acceptance by families, among other possible causes. We found that this gap has already been described in the literature, especially in relation to perinatal palliative care,10,11 with evidence that specific training programmes for emotional readiness in families and health care providers in relation to the diagnosis, prognosis, treatment options and psychosocial support in palliative care have achieved a significant increase in the number of patients in this age group that receive these services.
On the other hand, more than 15% of the patients that received palliative care were aged more than 14 years, and in this subset, one third were older than 19 years. This reflects the reality that some of the patients treated in paediatric units are not children, but adults whose condition or disease started during childhood or adolescence. This underscores the fact that PPCTs must be properly resourced, at both the clinical and administrative levels, to provide care for adult patients in select cases. Despite the importance of continuity of care in the transition from paediatric to adult services, the literature evinces significant gaps, and there is a dearth of data on mechanisms guaranteeing successful transition.12 Factors such as the dependence on paediatric care services, the lack of trust in other providers or inadequate communication have been described as perceived barriers to this transition by patients and families.13
Second, the results of the classification of the underlying diseases in our sample differed to some extent from the previous literature, which highlights the challenge posed by attempting such a classification. Contrary to the adult population, in which malignant diseases are predominant in the context of palliative care,14 the main diagnoses in paediatric patients receiving palliative care are neurologic diseases and genetic or congenital diseases, and cancer was the underlying disease in only 20% of the patients that received PPC in our study.10,15 However, when we analysed the subset of patients that received hospice care at the end of life, we found that patients with cancer accounted for 48% of the total.
When it came to the median duration of follow-up, we found shorter durations compared to the study by Siden et al.,11 who reported that 50% of the children were in the programme for at least 10 months.
This could mean that delayed admission to PPC programmes continues to be frequent. Often, the realization that death is inevitable lags behind disease progression, which in many cases results in an excessively aggressive approach to treatment. Multiple barriers to early referral by clinicians to PPC services have also been described.16 There may also be barriers related to the family due to the difficulties involved in entering a PPC programme, which entails a redefinition of treatment goals to focus on necessary care.17 In this regard, advance care planning is an essential component of palliative care that helps professionals reconcile the needs and best interests of the child and the goals and wishes of the family.18 Adequate advance care planning takes time, so efforts should be made to introduce palliative care early and not limit it to care at the end of life.19
Third, when it comes to the experienced symptoms, most studies to date have focused on specific symptoms in specific populations, and, in the case of patients with cancer, anorexia, fatigue, pain and dyspnoea are the most prevalent symptoms at the end of life.20–22 In contrast, compared to the adult population, few surveys have been conducted to analyse the symptoms at the end of life in children with life-limiting or life-threatening diseases. In our study, we found that care providers documented a mean of 5.5 symptoms per patient (standard deviation, 2.6), and only 2 symptoms were highly prevalent (found in more than 50% of the sample): dyspnoea and pain. The most common symptoms reported by Siden et al.11 were also dyspnoea (41%) and pain (22%), although with a lower frequency. In the study by Drake et al.,23 the mean number of documented symptoms was 11.1 (standard deviation, 5.6), while asthenia, behavioural changes, cutaneous changes, pain and oedema were highly prevalent, which suggests that symptom management may have been successful in our sample. Other studies where the symptoms at the end of life were reported by parents rather than health care professionals found significant disagreement between parental reports and the symptoms documented by clinicians. Thus, parents that stated that the physician was not actively involved in the of end-of-life care of the child were more likely to report that the child experienced pain. On the other hand, parents perceived the care of the dying child more positively when clinicians informed them clearly and unequivocally about what to expect at the end of life, including symptoms.24 In addition, follow-up of patients by PPCT was associated with an increased probability of parents describing the child as calm and peaceful in the last month of life.22 Thus, it is reasonable to deduce that end-of-life care includes preparing the family for the symptoms that will probably develop in the patients that may be most concerning to the parents. The follow-up of dying patients and their families by PPCTs allows clinicians to inform parents ahead of time of the symptoms most frequently associated with the disease at this stage, which can help relieve suffering in patients and parents and contribute to the future wellbeing of bereaved parents.25,26
Fourth, as expected, polypharmacy was documented in the week before death, with administration of a median of 6 drugs (IQR, 4) and frequent prescription of opioids and benzodiazepines. Still, this practice was substantially less frequent compared to the previous literature.23,27 These differences in relation to other studies may be explained by a large part of the patients dying in an intensive care unit, where polypharmacy is more frequent, which was the case of 66.7% of the sample in the study by Drake et al.,23 or a focus on pain management in children with cancer, as was the case of the study by Sirkiä et al.,27 in which most children (89%) required regular pain medication at the end of life. There is a generalised lack of evidence on symptom control in PPC,28 which indicates that little is known on the subject, and although in recent years there has been an increase in research on PPC, it has mainly focused on the organization of palliative care and not on symptom management.
Fifth, the place of death was the hospital in 57.9% of the patients and the home in 40.9%. Given that at present not every PPCT in Spain has the resources to provide home-based care, we must interpret this finding with caution. In PPC, home-based hospice care should be a basic service, as children and their families usually prefer to be home at the end of life, with evidence of better outcomes associated with hospital-at-home services.29,30 In countries like the United States, there is evidence that the number of patients that die at home is increasing with the advance of PPC services.31 In Germany, too, a study has found that in PPCTs that provide home-based care including a visiting 24/7 on-call service, 84% of the patients died at home.32 in contrast, a study conducted in the United Kingdom in a large sample found that 73% of the patients died in the hospital,33 which illustrates the variability in this aspect and its more than probable association with the services that are available. Further research is required to identified the factors that affect the place of death for patients with potentially fatal diseases and their families.34
To our knowledge, our study is the first ever conducted in Spain to analyse end-of-life care in paediatrics, the clinical characteristics of these patients and the delivery of care by PPCTs, but it has strengths and weaknesses that should be considered when interpreting its findings. As for its strengths, the cohort included patients managed in the period under study from 14 different geographical areas, which guarantees a good external validity. As for its weaknesses, the data collection was based on the review of health records, with the limitations that this method entails.35 In the future, research should include variables identified by the patients and families themselves, as they allowed a much more thorough evaluation of the provided care and unmet needs.36 In addition, the size of some of the subgroups based on the Feudtner classification was too small to detect differences, and in future studies, consecutive recruitment of patients should be performed to achieve a sufficient statistical power in the analysis of some of the subgroups.
Management by PPCTs is the ideal standard for the care of children and adolescents with life-threatening or life-limiting diseases and their families, in order to provide support and promote resilience and coping from the moment of diagnosis, especially in times of crisis and at the end of life.37 However, our data reflects a real-world scenario with potential deficiencies in access and inequality in the delivery of care in certain segments of the paediatric population.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Peláez Cantero MJ, Morales Asencio JM, Navarro Marchena L, Velázquez González MR, Sánchez Echàniz J, Rubio Ortega L, Martino Alba R. El final de vida en pacientes atendidos por equipos de cuidados paliativos pediátricos. Estudio observacional multicéntrico, Anales de Pediatría. 2022;96:394–401.