Tobacco smoking may cause adverse effects during pregnancy and postpartum. The aim of this study was to evaluate several repercussions of active and/or passive smoking by the mother at home, during pregnancy, at delivery, as well as for the newborn baby and breastfeeding, including the effect of quitting smoking in the first trimester of pregnancy.
MethodsA prospective longitudinal study was carried out with a sample of 800 pregnant women. Four evaluations were made: first and third trimester of pregnancy, and 2 and 6/8 months postpartum. Sociodemographic, obstetric, health and tobacco consumption details were collected, with biochemical tests being performed to confirm the self-reported abstinence.
ResultsBeing an active and passive smoker predicted suffering complications in pregnancy (OR 2.50; 95% CI; 1.42–4.35) and delivery (OR 3.10; 95% CI; 1.75–5.51), and also tended not to breastfeed (OR 2.44; 95% CI; 1.35–4.42). Being an active smoker predicted complications at childbirth (OR 5.58; 95% CI; 2.64–7.77), for the baby (OR 3.77; 95% CI; 1.53–9.36) and not breastfeeding at 2 (OR 25.73; 95% CI; 11.95–55.40), and 6/8 months postpartum (OR 6.61; 95% CI; 3.21–13.58). Being a passive smoker reduces the intend to breastfeed (OR 1.81; 95% CI; 1.11–2.95), and the practice of breastfeeding at 2 months postpartum (OR 1.94; 95% CI; 1.11–3.37). Women who quit smoking are less likely to suffer complications in pregnancy and childbirth, and are more likely to attend antenatal and breastfeeding classes, and to have babies with higher birth weight.
ConclusionsActive and passive tobacco consumption has significant implications during pregnancy and postpartum period. Smoking cessation at the beginning of pregnancy reverses much of these effects.
Fumar puede provocar efectos adversos durante el embarazo y el postparto. El objetivo de este estudio fue evaluar diversas repercusiones que puede acarrear el consumo de tabaco materno activo y/o pasivo en el hogar, en el embarazo, parto, recién nacido y en la lactancia materna, así como el efecto del abandono del tabaco en el primer trimestre de embarazo.
MétodoEstudio longitudinal prospectivo realizado con una muestra de 800 mujeres embarazadas. Se realizaron cuatro evaluaciones: primer y tercer trimestre de embarazo y 2 y 6/8 meses postparto. Se recogió información sociodemográfica, obstétrica, relacionada con la salud y con el consumo de tabaco, y se validó bioquímicamente el autoinforme de abstinencia.
ResultadosSer fumadora activa y pasiva predice sufrir complicaciones en el embarazo (OR 2,50; IC 1,42–4,35) y el parto (OR 3,10; IC 1,75–5,51) y tener la intención de no amamantar (OR 2,44; IC 1,35–4,42). Ser fumadora activa predice presentar complicaciones en el parto (OR 5,58; IC 2,64–7,77), el bebé (OR 3,77; IC 1,53–9,36) y no practicar lactancia materna a los 2 (OR 25,73; IC 11,95–55,40) y 6/8 meses postparto (OR 6,61; IC 3,21–13,58). Ser fumadora pasiva reduce la intención de amamantar (OR 1,81; IC 1,11–2,95) y la lactancia a los 2 meses postparto (OR 1,94; IC 1,11–3,37). Las mujeres que dejan de fumar presentan una menor probabilidad de sufrir complicaciones en el embarazo y el parto, mayor probabilidad de asistir a las clases preparto y de lactancia, y mayor peso de los recién nacidos.
ConclusionesEl consumo de tabaco tanto activo como pasivo conlleva importantes repercusiones tanto en el embarazo como en el postparto. Dejar de fumar al inicio del embarazo revierte estos efectos.
Although pregnancy is among the strongest motivators to quit smoking,1,2 a substantial percentage of women still smoke during gestation, and Spain is one of the European countries where smoking during pregnancy is most prevalent.3 An important aspect to consider is that women that continue to smoke during pregnancy reduce their consumption of tobacco with the belief that this can minimise or eliminate risk to the foetus, despite evidence that there is no threshold under which tobacco exposure is risk-free.4
Tobacco consumption in the perinatal period is one of the few modifiable risk factors associated with adverse outcomes. Tobacco consumption during conception and pregnancy is associated with an increased risk of placental disease,5,6 ectopic pregnancy7,8 and spontaneous miscarriage.8,9 It also carries risks to the health of the baby. Preterm birth10–12 and low birth weight or small for gestational age are among the most frequent consequences.13 Babies born to mothers that smoke weight approximately 150 to 250 g less than babies born to mothers that do not smoke.12–15 There is also an increased risk of perinatal mortality and developmental delay.8,16 In contrast, quitting smoking before or during pregnancy reduces these risks.12
In the postpartum period, in case of breastfeeding, the infant is exposed to nicotine through breast milk, which may cause nausea and diarrhoea.17 Many smoking mothers choose not to breastfeed18,19 to prevent potential deleterious effects on the baby, thereby relinquishing the benefits of breastfeeding.20
On the other hand, sharing the immediate environment with a smoker exposes nonsmokers to tobacco smoke. This involuntary or passive exposure to tobacco also increases the risk of disease.21 Infants may become passive smokers due to tobacco consumption by either parent or other household members, which is associated with an increased risk of, among others, respiratory diseases.7
While there is ample evidence in the literature on the risks associated with maternal smoking during pregnancy,5–15 few studies have focused on the specific effects of passive exposure due to tobacco consumption by the partner of the mother and/or other household members, especially in the postpartum period. Tobacco consumption by the partner is associated with an increased probability of maternal smoking during pregnancy,2,22 but there is a dearth of data on the impact of partner smoking, in isolation or in association with maternal smoking, on the development of complications during pregnancy and/or childbirth and in the infant, as well as on breastfeeding. Therefore, it would be important to analyse the isolated and combined effects of tobacco consumption by the mother and her partner.
In Spain, 2 studies have assessed the impact on pregnancy and birth outcomes of maternal active and passive smoking during pregnancy. The first one23 assessed the impact on the infant but did not analyse the benefits of quitting smoking soon after detection of the pregnancy. The second12 focused on the impact of maternal active and passive smoking during pregnancy specifically on birth weight and preterm birth.
We conducted a prospective longitudinal study with follow-up of a large sample of women during pregnancy and the postpartum period with the aim of analysing: (a) the impact of maternal active smoking and/or passive smoking in the household on pregnancy, childbirth, infant and breastfeeding outcomes and (b) the impact of quitting tobacco consumption in the first trimester of the pregnancy.
Material and methodsParticipantsThe study population consisted of women going through pregnancy and the postpartum period. The sample included 800 women followed up from the first trimester of gestation through 6–8 months postpartum. The inclusion criteria were: age 18 years or greater, ability to speak and read Spanish, gestation of 20 weeks or less at the time of recruitment and not changing smoking status during the follow-up, that is, not experiencing relapses or quitting smoking after the first trimester.
Study design and procedureWe conducted a prospective longitudinal study with assessments at 4 time points: first and third trimester of pregnancy and 2 and 6/8 months postpartum. We recruited 901 women during the first trimester of pregnancy in 10 primary care centres in the provinces of A Coruña and Pontevedra in Galicia (Spain) through consecutive sampling. Participation was voluntary and not remunerated. We obtained written informed consent from all participants. At the time assessments were due, appointments were scheduled by telephone. Participants chose the date, time and setting of these visits, which were conducted in the home of the participant in 80% of cases. The mean duration of the individual interviews with participants at each follow-up time point was of approximately 25 min. Recruitment opened in December 2012 and closed in March 2015.
The study was conducted in adherence with the Declaration of Helsinki and approved by the Directorate General of Research, Education and Innovation and the Clinical Research Ethics Committee of Galicia.
Assessment toolsWe developed 4 questionnaires ad hoc (one for each assessment waves) to collect data on sociodemographic characteristics, tobacco consumption and obstetrics and health-related variables: complications during pregnancy (bleeding, threatened miscarriage or preterm birth, gestational diabetes, placental abnormalities, foetal growth abnormalities), during childbirth (haemorrhage, abnormal foetal presentation, failure to labour, foetal distress) and foetal/infant problems (respiratory, gastrointestinal).
Smoking status was self-reported and verified at each timepoint with biochemical tests. In the first trimester, we used the urine cotinine test, and at all other timepoints we used measured carbon monoxide (CO) in expired air (piCO Smokerlyzer monitor).
We collected data on maternal current smoking status and passive exposure to smoke at home, that is, whether the mother lived with other smokers, be it her partner or other household members. If participants reported smoking, we asked how many cigarettes they used to smoke before getting pregnant and how many they smoked currently. We created 2 distinct classifications based on maternal smoking status and the degree of maternal exposure to tobacco smoke in the household.
We classified participants into 3 groups based on smoking status, defined based on self-reporting and the results of biochemical testing. Nonsmoker: reported never having smoked or having quit at least 3 months before conception. Continuous abstinent: reported having quit in the first trimester of pregnancy and remaining abstinent through pregnancy and the postpartum period (confirmed by biochemical testing). Continuous smoker maintained tobacco use through the follow-up period.
We also established 4 groups based on exposure to tobacco smoke in the home: smoke-free home if no one smoked in the household; active smoker if the participant was the only smoker; passive smoker if other household members smoked but the participant did not; active and passive smoker if both the participant and at least another household member smoked.
Data analysisWe performed the statistical analysis with the software IBM SPSS Statistics for Windows, version 20. Statistical significance was defined as a p-value of less than 0.05. We assessed differences between groups by means of the χ2 in case of categorical variables and analysis of variance (ANOVA) in case of continuous variables. We calculated the effect size (0–1) to assess the strength of the association using Cramer’s V (categorical variables) or the η2 (continuous variables). We also carried out post hoc tests to detect between which groups there are differences. We performed binary logistic regression analyses for each categorical variable to determine which of the household exposure categories was the best predictor of each of the outcomes under study. We also fit a linear regression model in which birth weight was the continuous dependent variable.
ResultsSample characteristicsOf the 901 women initially recruited, 28 had miscarriage, we were unable to get in touch with 29, 4 chose to drop from the study and 40 changed their smoking status during the follow-up. Thus, the final sample included 800 women. Participants were aged 18 to 46 years (mean, 32.7 years; standard deviation [SD], 4.37). Most were aged more than 30 years (71.6%), were married or lived with a partner (83.5%), were primiparous (65.8%), had a university degree (53.8%) and were employed (71.0%) (Table 1).
Sociodemographic characteristics in relation to active and passive smoking in the sample under study.
| Continuous smoker, n = 133 | Continuous abstinent, n = 85 | Nonsmoker, n = 582 | χ2 | Cramer’s V | Total, N = 800 | ||
|---|---|---|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | ||||
| Age | ≤30 years | 32.3% (43) | 31.8% (27) | 27.0% (157) | 2.07 | 28.4% (227) | |
| >30 years | 67.7% (90) | 68.2% (58) | 73.0% (425) | 71.6% (573) | |||
| Marital status | Without a partner | 24.8% (33) | 18.8% (16) | 14.3% (83) | 9.12* | 0.107 | 16.5% (132) |
| Had a partner | 75.2% (100) | 81.2% (69) | 85.7% (499) | 83.5% (668) | |||
| Number of children | Primiparous | 68.4% (91) | 78.8% (67) | 63.2% (368) | 8.51* | 0.103 | 65.8% (526) |
| Multiparous | 31.6% (42) | 21.2% (18) | 36.8% (214) | 34.3% (274) | |||
| Educational attainment | Less than university degree | 75.2% (100) | 47.1% (40) | 39.5% (230) | 55.43*** | 0.263 | 46.3% (370) |
| University degree | 24.8% (33) | 52.9% (45) | 60.5% (352) | 53.8% (430) | |||
| Employment status | Not employed | 38.3% (51) | 25.9% (22) | 27.3% (159) | 6.84* | 0.092 | 29.0% (232) |
| Employed | 61.7% (82) | 74.1% (63) | 72.7% (423) | 71.0% (568) | |||
| Income | ≤2000€ | 73.6% (78) | 69.3% (52) | 59.5% (322) | 9.11* | 0.112 | 62.6% (452) |
| >2000€ | 26.4% (28) | 30.7% (23) | 40.5% (219) | 37.4% (270) |
| Smoke-free home, n = 463 | Active smoker, n = 37 | Passive smoker, n = 205 | Active and passive smoker, n = 95 | χ2 | Cramer’s V | ||
|---|---|---|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | ||||
| Age | ≤30 years | 25.5% (118) | 18.9% (7) | 31.7% (65) | 38.9% (37) | 9.87* | 0.111 |
| >30 years | 74.5% (345) | 81.1% (30) | 68.3% (140) | 61.1% (58) | |||
| Marital status | Without a partner | 13.0% (60) | 18.9% (7) | 19.0% (39) | 27.4% (26) | 13.46* | 0.130 |
| Had a partner | 87.0% (403) | 81.1% (30) | 81.0% (166) | 72.6% (69) | |||
| Number of children | Primiparous | 61.1% (283) | 62.2% (23) | 74.1% (152) | 71.6% (68) | 12.46* | 0.125 |
| Multiparous | 38.9% (180) | 37.8% (14) | 25.9% (53) | 28.4% (27) | |||
| Educational attainment | Less than university degree | 34.1% (158) | 67.6% (25) | 54.6% (112) | 78.9% (75) | 80.79*** | 0.318 |
| University degree | 65.9% (305) | 32.4% (12) | 45.4% (93) | 21.1% (20) | |||
| Employment status | Not employed | 25.3% (117) | 29.7% (11) | 31.2% (64) | 42.1% (40) | 11.55* | 0.120 |
| Employed | 74.7% (346) | 70.3% (26) | 68.8% (141) | 57.9% (55) | |||
| Income | ≤2000€ | 58.0% (251) | 63.0% (17) | 66.8% (123) | 78.2% (61) | 13.50* | 0.137 |
| >2000€ | 42.0% (182) | 37.0% (10) | 33.2% (61) | 21.8% (17) |
The percentage of participants continuous smokers was 16.6% (95% confidence interval [CI], 14.3%–19.0%) and the percentage continuous abstinents was 10.6% (95% CI, 8.8%–12.8%). In the continuous smoker group, the mean self-reported number of cigarettes smoked per day was 5.8 (SD, 3.58) in the first trimester, 4.2 (SD, 1.91) in the third trimester, 7.6 (SD, 3.60) at 2 months postpartum and 8.6 (SD, 3.67) at 6–8 months postpartum. In addition, 60.9% (n = 81) reported that they had decreased their consumption in the past year by a mean of 10.4 cigarettes (SD, 4.67).
When it came to exposure to tobacco smoke in the household, 37.5% (n = 300) reported living with smokers, which was the partner in 84.6% of cases (n = 253).
Impact of maternal tobacco consumption status on pregnancy, childbirth and infant outcomesIn the continuous smoker group, participants were more likely to plan not to breastfeed (p = .016), have assisted births (p = .018) and have babies with health problems (p = .043), compared to the nonsmoker and continuous abstinent groups (Table 2).
Impact of maternal smoking status on pregnancy, childbirth and infant health.
| (1) Continuous smoker, n = 133 | (2) Continuous abstinent, n = 85 | (3) Nonsmoker, n = 582 | χ2 | (Cramer’s V) | Group comparison | Total, N = 800 | ||
|---|---|---|---|---|---|---|---|---|
| Pregnancy and childbirth | % (n) | % (n) | % (n) | % (n) | ||||
| Attended childbirth education | Yes | 33.8% (45) | 63.5% (54) | 54.6% (318) | 23.73*** | (0.172) | 1 < 2,3 | 52.1% (417) |
| No | 66.2% (88) | 36.5% (31) | 45.4% (264) | 47.9% (383) | ||||
| Intended to breastfeed | Yes | 81.2% (108) | 83.5% (71) | 89.5% (521) | 8.22* | (0.101) | 1 < 3 | 87.5% (700) |
| No | 18.8% (25) | 16.5% (14) | 10.5% (61) | 12.5% (100) | ||||
| Complications during pregnancy | Yes | 21.1% (28) | 8.2% (7) | 12.4% (72) | 9.21* | (0.107) | 1 > 2,3 | 13.4% (107) |
| No | 78.9% (105) | 91.8% (78) | 87.6% (510) | 86.6% (693) | ||||
| Complications during delivery | Yes | 26.3% (35) | 12.9% (11) | 8.4% (8.4) | 33.24*** | (0.204) | 1 > 2,3 | 11.9% (95) |
| No | 73.7% (98) | 87.1% (74) | 91.6% (533) | 88.1% (705) | ||||
| Type of delivery | ||||||||
| Normal | 60.9% (81) | 71.8% (61) | 73.7% (429) | 11.90* | (0.086) | 1 < 3 | 71.4% (571) | |
| Caesarean | 15.8% (21) | 16.5% (14) | 13.4% (78) | 14.1% (113) | ||||
| Forceps/vacuum | 23.3% (31) | 11.8% (10) | 12.9% (75) | 14.5% (116) | ||||
| Newborn health | ||||||||
| Complications | Yes | 12.0% (16) | 9.4% (8) | 6.0% (35) | 6.31* | (0.089) | 1 > 3 | 7.4% (59) |
| No | 88.0% (117) | 90.6% (77) | 94.0% (547) | 92.6% (741) | ||||
| Preterm birth | Yes | 3.8% (5) | 4.7% (4) | 4.0% (23) | 0.13 | 4.0% (32) | ||
| No | 96.2% (128) | 95.3% (81) | 96.0% (559) | 96.0% (768 | ||||
| Low birth weight | Yes | 8.3% (11) | 5.9% (5) | 4.5% (26) | 3.23 | 5.3% (42) | ||
| No | 91.7% (122) | 94.1% (80) | 95.5% (556) | 94.7% (758) | ||||
| Breastfeeding at 2 months post birth | Yes | 48.1% (64) | 77.6% (66) | 93.6% (545) | 173.44*** | (0.466) | 1 < 2,32 < 3 | 84.4% (675) |
| No | 51.9% (69) | 22.4% (19) | 6.4% (37) | 15.6% (125) | ||||
| Breastfeeding at 6–8 months post birth | Yes | 39.8% (53) | 70.6% (60) | 77.0% (448) | 71.24*** | (0.298) | 1 < 2,3 | 70.1% (561) |
| No | 60.2% (80) | 29.4% (25) | 23.0% (134) | 29.9% (239) |
On the other hand, women that quit smoking in the first trimester and remained abstinent had fewer complications during pregnancy (p = .010) and childbirth (p < .001), and a greater percentage in this group attended childbirth education classes (p < .001), were breastfeeding at 2 months postpartum (p < .001) and were breastfeeding at 6-8 months post partum (p < .001). The only significant difference between the continuously abstinent and nonsmoker groups was a lower percentage of breastfeeding at 2 months post partum in the former (p < .001).
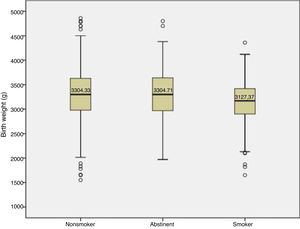
We found differences in the mean birth weight of babies between smoking status groups (F = 5.95; p = .003; η2 = 0.015) (Fig. 1). Specifically, babies of nonsmokers (p = .002) and continuous abstinent mothers (p = .036) had greater birth weights compared to babies of continuous smoker mothers (3304.33 vs 3304.71 vs 3127.37 g). We found no significant differences in the birth weight of babies of nonsmokers versus continuous abstinent mothers.
Impact of maternal active and/or passive smoking on pregnancy, childbirth and infant outcomesWomen who were both active and passive smokers were less likely to attend childbirth education classes (p < .001) and more likely to have complications during pregnancy (p = .012) and delivery (p < .001) and have assisted births (p = .003) (Table 3). Women who were passive smokers were less likely to intend to breastfeed (p = .009) and to be breastfeeding at 2 months postpartum (p < .001) compared to women in smoke-free homes. Active smokers were less likely to be breastfeeding at 6 to 8 months postpartum compared to women in smoke-free homes or passive smokers (p < .001).
Impact of maternal active and/or passive smoking in the household on pregnancy, childbirth and newborn health.
| (1) Smoke-free home, % (n) | (2) Active smoker, % (n) | (3) Passive smoker, % (n) | (4) Active and passive smoker, % (n) | χ2 | Cramer’s V | Group comparison | ||
|---|---|---|---|---|---|---|---|---|
| Pregnancy and childbirth | 57.9% (463) | 4.6% (37) | 25.6% (205) | 11.9% (95) | ||||
| Attended childbirth education | Yes | 54.4% (252) | 37.8% (14) | 58.5% (120) | 32.6% (31) | 21.86*** | 0.165 | 4 < 1,3 |
| No | 45.6% (211) | 62.2% (23) | 41.5% (85) | 67.4% (64) | ||||
| Intended to breastfeed | Yes | 90.7% (420) | 83.8% (31) | 84.4% (173) | 80.0% (76) | 11.54* | 0.120 | 1 > 2,3,4 |
| No | 9.3% (43) | 16.2% (6) | 15.6% (32) | 20.0% (19) | ||||
| Complications during pregnancy | Yes | 10.8% (50) | 16.2% (6) | 14.1% (29) | 23.2% (22) | 10.86* | 0.117 | 4 > 1 |
| No | 89.2% (413) | 83.8% (31) | 85.9% (176) | 76.8% (73) | ||||
| Complications during delivery | Yes | 8.9% (41) | 35.1% (13) | 9.3% (19) | 23.2% (22) | 36.05*** | 0.212 | 4 > 1,3 |
| 2 > 3 | ||||||||
| No | 91.1% (422) | 64.9% (24) | 90.7% (186) | 76.8% (73) | ||||
| Type of delivery | Normal | 76.5% (354) | 54.1% (20) | 66.8% (137) | 63.2% (60) | 19.65* | 0.111 | 4 < 1,3 |
| Caesarean | 12.7% (59) | 18.9% (7) | 16.1% (33) | 14.7% (14) | 4 > 2 | |||
| Instrumental | 10.8% (50) | 27.0% (10) | 17.1% (35) | 22.1% (21) | ||||
| Newborn health | ||||||||
| Complications | Yes | 5.8% (27) | 18.9% (7) | 7.8% (16) | 9.5% (9) | 9.50* | 0.109 | 2 > 1 |
| No | 94.2% (436) | 81.1% (30) | 92.2% (189) | 90.5% (86) | ||||
| Preterm birth | Yes | 4.5% (21) | 5.4% (2) | 2.9% (6) | 3.2% (3) | 1.33 | ||
| No | 95.5% (442) | 94.6% (35) | 97.1% (199) | 96.8% (92) | ||||
| Low birth weight | Yes | 4.1% (19) | 10.8% (4) | 5.9% (12) | 7.4% (7) | 4.53 | ||
| No | 95.9% (444) | 89.2% (33) | 94.1% (193) | 92.6% (88) | ||||
| Breastfeeding (2 months post birth) | Yes | 93.3% (432) | 35.1% (13) | 87.8% (180) | 52.6% (50) | 170.49*** | 0.462 | 1 > 2,3,4 |
| 3 > 2,4 | ||||||||
| No | 6.7% (31) | 64.8% (24) | 12.2% (25) | 47.4% (45) | ||||
| Breastfeeding (6–8 months post birth) | Yes | 76.0% (352) | 32.4% (12) | 76.1% (156) | 43.2% (41) | 69.26*** | 0.294 | 1 > 2,4 |
| 3 > 2,4 | ||||||||
| No | 24.0% (111) | 67.6% (25) | 23.9% (49) | 56.8% (54) |
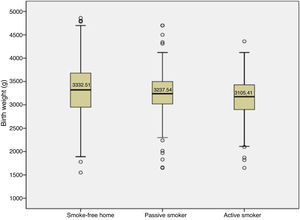
Babies of smoking mothers were more likely to have health problems compared to babies born to mothers in smoke-free homes (p = .023). Babies had greater birth weights in the smoke-free home group (F = 5.25; p = .001; η2 = 0.019), with significant differences compared to babies whose mothers were both active and passive smokers (p = .010) (Fig. 2).
Predictors of outcomes associated with maternal active and/or passive smokingThe combined active and passive smoker status was the strongest predictor of complications during pregnancy (odds ratio [OR] = 2.50) and not planning to breastfeed (OR = 2.44) (Tables 4 and 5).
Maternal active and/or passive smoking as a predictor of complications during pregnancy, during delivery and in the newborn.
| Complications in pregnancy | Complications in delivery | Health problems in newborn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Wald | p | OR (95% CI) | B | Wald | p | OR (95% CI) | B | Wald | p | OR (95% CI) | |
| Smoke-free home (reference group) | 10.45 | .015 | 31.73 | <.001 | 8.68 | .034 | ||||||
| Passive smoker | 0.31 | 1.52 | .218 | 1.36 (0.83–2.22) | 0.05 | 0.03 | .863 | 1.05 (0.59–1.86) | 0.31 | 0.91 | .339 | 1.37 (0.72–2.60) |
| Active smoker | 0.47 | 1.00 | .319 | 1.61 (0.64–4.00) | 1.72 | 20.31 | <.001 | 5.58 (2.64–7.77) | 1.33 | 8.17 | .004 | 3.77 (1.52–9.36) |
| Active and passive smoker | 0.91 | 10.20 | .001 | 2.50 (1.42–4.35) | 1.13 | 14.92 | <.001 | 3.10 (1.75–5.51) | 0.53 | 1.70 | .192 | 1.69 (0.77–3.72) |
| Constant | 2.11 | 198.83 | <.001 | −2.33 | 203.12 | <.001 | 0.097 | −2.78 | 196.75 | <.001 | 0.062 | |
Maternal active and/or passive smoking as a predictor of breastfeeding.
| Did not intend to breastfeed | Did not breastfeed at 2 months post partum | Did not breastfeed at 6–8 months post partum | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Wald | p | OR (95% CI) | B | Wald | p | OR (95% CI) | B | Wald | p | OR (95% CI) | |
| Smoke-free home (reference group) | 11.17 | .011 | 125.18 | <.001 | 61.18 | <.001 | ||||||
| Passive smoker | 0.59 | 5.58 | .018 | 1.81 (1.11–2.95) | 0.66 | 5.44 | .020 | 1.94 (1.11–3.37) | −<0.01 | <0.01 | .984 | 1.00 (0.68–1.46) |
| Active smoker | 0.64 | 1.81 | .179 | 1.89 (0.75–4.79) | 3.25 | 68.86 | <. 001 | 25.73 (11.95–55.40) | 1.89 | 26.37 | <.001 | 6.61 (3.21–13.58) |
| Active and passive smoker | 0.89 | 8.72 | .003 | 2.44 (1.35–4.42) | 2.53 | 83.29 | <.001 | 12.54 (7.29–21.59) | 1.43 | 37.32 | <.001 | 4.18 (2.64–6.61) |
| Constant | −2.28 | 202.60 | <.001 | 0.10 | −2.63 | 200.74 | <.001 | .072 | −1.15 | 112.40 | <.001 | 0.32 |
The active smoking status was the best predictor of complications during delivery (OR = 5.58), health problems in the baby (OR = 3.77), and absence of breastfeeding at 2 months postpartum (OR = 25.73) and 6 to 8 months postpartum (OR = 6.61).
Passive smoker status was not a significant predictor of complications during pregnancy or delivery or health problems in the baby. However, it was associated with a lesser probability of intending to breastfeed (OR = 1.81) and of breastfeeding at 2 months postpartum (OR = 1.94).
We found that birth weight decreased incrementally and significantly with each smoking status category (beta = –0.13; t = –3.80, p < .001).
DiscussionOur study analysed different potential repercussions of active and/or passive maternal smoking on pregnancy, childbirth, newborn health and breastfeeding, as well as the effect of quitting smoking in the first trimester of pregnancy. While most smokers reported that they had reduced tobacco consumption to fewer than 10 cigarettes a day due to their pregnancy, we found an association between tobacco consumption and a higher incidence of complications as well as a lower prevalence of breastfeeding. This is important, since, as previously reported in the literature,4,6,9 our findings evince that even smoking in small amounts (fewer than 10 cigarettes a day) has deleterious effects.
On the other hand, maternal smoking can cause health problems in the newborn infant, as carbon monoxide may result in abnormal placental vascularization, placental hypertrophy and/or local hypoxia, reducing uterine blood flow and increasing the risk of complications and intrauterine growth restriction.5 In addition, nicotine is a vasoconstrictor and may cause withdrawal in the newborn infant.24 Our study did not find evidence of an impact in terms of preterm birth or low birth weight, contrary to previous studies,10–12 possibly due to the low frequency of preterm birth (4.0%) and low birth weight (5.3%). However, when we compared birth weight in grams, we did find that a lower mean in babies born to mothers that smoked (by 227 g).14,15
In addition, our findings showed that quitting smoking at the beginning of the pregnancy, that is, early cessation, and maintaining abstinence during the perinatal period, reduced risks to levels similar to those observed in nonsmokers. For example, compared to participants that smoked, participants that quit smoking and remained continuously abstinent had fewer complications during pregnancy and delivery and were more likely to attend childbirth education classes and breastfeed at 2 months and 6–8 months postpartum.
Breastfeeding offers considerable health benefits to both mother and child. Breastfed children are at lower risk of developing infectious diseases, diabetes, overweight and obesity.25 When it comes to mothers, breastfeeding reduces the risk of breast and ovarian cancer, reduces blood pressure26 and has mental health benefits, as it decreases the stress response27 and reinforces the mother-child bond.28 Therefore, breastfeeding can have a protective effect against smoking relapse and promote abstinence. In fact, our data revealed a positive correlation between breastfeeding and maintenance of smoking cessation.20,29
Another important objective in our study was to assess the impact of passive smoking in the household. We found that 37.5% of participants lived with at least one smoker, in most cases her spouse or partner (84.6%). The group that exhibited the least negative repercussions (fewer pregnancy complications and health problems in the baby and greater prevalence of breastfeeding) was the smoke-free home group, while the presence of even one smoker in the home was associated with an increased risk of adverse health outcomes. We ought to highlight that passive smoking had an impact on breastfeeding. We also found that exposure to smoke had a cumulative effect, as complications increased progressively from the smoke-free home group to the passive smoker group, active smoker group and finally the active and passive smoker group.
Certain limitations must be taken into account when it comes to the interpretation of our findings. First, the outcome variables used to assess the presence of complications were dichotomous, and it will be important for future studies to analyse the number and type of complications, rather than merely whether they develop or not. In addition, the size of some of the groups was small, which made it difficult to find statistically significant differences. Also, some subsets of the population with specific sociodemographic characteristics, for instance with a certain educational attainment, may have been overrepresented in our sample compared to the study population. Lastly, the timing of the fourth assessment was not homogeneous due to the sample size and the performance of individual interviews, so that it took place between 6 and 8 months postpartum, which could be a confounder in the assessment of breastfeeding discontinuation. Despite these limitations, there were also significant strengths to our study, as it is the first in Spain to assess the impact of tobacco consumption by not only the mother but also her partner or other household on pregnancy, childbirth, the postpartum period and, specifically, on breastfeeding. It was a prospective longitudinal study that evaluated a large number of women throughout pregnancy and up to 6–8 months postpartum, which allowed us to assess causality. Furthermore, we verified self-reported smoking cessation with biochemical tests. This is particularly relevant in this subset of the population on account of the considerable pressure placed on women to not smoke during pregnancy, which may lead some of them to hide their tobacco consumption.30 On the other hand, when it came to active smoking, we compared 3 groups (continuous smokers, nonsmokers and continuous abstinents), which allowed us to assess the impact of smoking cessation at the beginning of the pregnancy (for instance, birth weights improved and were not significantly different from those observed in the nonsmoker group).
Our findings evince some of the risks of maternal active smoking or passive smoking due to exposure to second-hand smoke from other smokers in the household during pregnancy and the postpartum period. This is very relevant from a clinical standpoint, as maternal passive smoking is a neglected variable in clinical practice, that is, if the mother reports not smoking, it is believed that she and her baby are not at risk of de deleterious effects of tobacco. Now we are aware that it is also important to ask about tobacco consumption in other household members, as exposure to second-hand smoke is also associated with adverse effects. Understanding the impact of active and/or passive smoking on pregnancy, childbirth and postpartum period outcomes can help improve the guidance provided to pregnant women on the subject of smoking cessation. To this end, it is essential that all health professionals involved in prenatal and postpartum care delivery promote smoking cessation, maintenance of abstinence and a smoke-free home environment. We observed that the adverse effects of active and passive smoking were not limited to the pregnancy, but continued in the postpartum period, even affecting breastfeeding. When it comes to smoking cessation, the postpartum period is associated with a high risk of relapse, which increases health risks for mothers and infants and reduces the probability of breastfeeding. Adequate care during the postpartum period focusing not only on the health of the infant but also on family habits could reduce the risk and the associated negative repercussions. In this regard, paediatric professionals could play a key role in improving maternal and infant health through measures like the promotion of breastfeeding or smoking cessation and relapse prevention.
Until now, we were aware of the need to offer support to pregnant women and their partners to quit smoking.22 Our findings evince the importance of extending this support to other household members and to promote breastfeeding, as the latter is associated with continuous abstinence.
FundingThe study did not receive any form of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Míguez MC, Pereira B. Effects of active and/or passive smoking during pregnancy and the postpartum period. An Pediatr (Barc). 2021;95:222–232.