Environmental exposure to tobacco increases the risk of respiratory disease in infants. However, the impact of maternal smoking on the development of acute bronchiolitis has hardly been assessed. The aim of this study was to determine the incidence of acute bronchiolitis and to analyse the effect of prenatal and postnatal maternal smoking on the development of this disease.
Patients and methodsA prospective, observational study was performed on healthy newborns from a third level hospital born between October 2015 and February 2016. Questionnaires were completed by the mothers at discharge from maternity and followed-up for two years. These collected information about prenatal and postnatal smoking, lifestyle, family and personal history, and the development of bronchiolitis. A bivariate and multivariate logistic regression analysis was performed.
ResultsA total of 223 newborns were included, of whom 13.9% were exposed to tobacco smoking during gestation, 21.4% in the postnatal period, and 12.4% in both times. The incidence of bronchiolitis was 28.7% at one year of life, and 34.5% at two years. The multivariate analysis demonstrated that the prenatal and postnatal exposure to tobacco is an independent risk factor for the development of bronchiolitis (OR 4.38; 95% CI; 1.63–11.76), while prolonged breastfeeding is a protective factor (OR 0.13; 95% CI; 0.04–0.48). Other factors that were statistically significant were: atopic dermatitis (OR 2.91; 95% CI; 1.26–6.73), and gestational age (OR 1.42; 95% CI; 1.08–1.88).
ConclusionsChildren exposed to prenatal and postnatal maternal smoking have a higher risk of suffering bronchiolitis. Reducing the smoking habit in women that intend to become pregnant must be a priority in preventive medicine.
La exposición ambiental al tabaco incrementa el riesgo de patología respiratoria en la infancia. Sin embargo, el impacto del tabaquismo materno en el desarrollo de bronquiolitis aguda ha sido escasamente evaluado. El objetivo del estudio fue determinar la incidencia de bronquiolitis aguda y analizar el efecto del tabaquismo materno prenatal y postnatal en el desarrollo de esta patología.
Pacientes y métodosEstudio observacional, prospectivo, que incluyó recién nacidos sanos de un hospital terciario entre octubre-2015 y febrero-2016. Se realizaron encuestas a las madres al alta de maternidad y seguimiento durante dos años, que recogieron información sobre tabaquismo prenatal y postnatal, estilo de vida, antecedentes familiares y personales, y desarrollo de bronquiolitis. Se realizó análisis de regresión logística bivariante y multivariante.
ResultadosSe incluyeron 223 recién nacidos, 13,9% estuvieron expuestos a tabaquismo durante la gestación, 21,4% en período postnatal y 12,4% en ambos momentos. La incidencia de bronquiolitis fue de 28,7% al año de vida y de 34,5% a los dos años. El análisis multivariante demostró que la exposición prenatal y postnatal al tabaco es un factor de riesgo independiente para el desarrollo de bronquiolitis (OR 4,38; IC95% 1,63–11,76), mientras que la lactancia materna prolongada es un factor protector (OR 0,13; IC95% 0,04–0,48). Otros factores que resultaron estadísticamente significativos fueron: dermatitis atópica (OR 2,91; IC95% 1,26–6,73) y edad gestacional (OR 1,42; IC95% 1,08–1,88).
ConclusionesLos niños expuestos a tabaquismo materno prenatal y postnatal presentan un mayor riesgo de padecer bronquiolitis. La disminución del hábito tabáquico en mujeres con intención de embarazo debe ser una prioridad en medicina preventiva.
The use of tobacco during pregnancy is a significant public health problem on account of its deleterious impact on gestation and foetal development.1,2 Although its noxious effects are well-known, tobacco use continues to be frequent among pregnant women, with variations between geographical regions. In Europe, the prevalence of tobacco use during pregnancy is of approximately 20%. In Spain, between 30% and 43% of women smoke at the time of conception and between 13% and 25% continue to smoke throughout the pregnancy.3
The impact of intrauterine exposure to tobacco manifest in the gestational age at birth and throughout childhood. Maternal smoking during pregnancy can affect postnatal pulmonary function in the child, and this may lead to an increased risk of respiratory disease.4–7 However, since 90% of women that smoke during pregnancy continue to smoke in the years that follow, it is not easy to differentiate between the adverse effects on the child that result from the impact of intrauterine exposure to tobacco on foetal development versus postnatal exposure to tobacco smoke.8–10
Several studies have provided evidence on the role of passive smoking post birth as a risk factor for respiratory disease. Children of parents that smoke are at increased risk of contracting respiratory infections and there is also a positive correlation between passive exposure to tobacco at early ages and the subsequent development of bronchial hyperresponsiveness.11–13
Lower respiratory tract infections are frequent in childhood and in some cases severe, and they constitute a significant burden at every level of care with a substantial economic impact on public health systems.14,15 Acute bronchiolitis (AB) is the main reason for admission due to lower respiratory tract infection in children under 2 years of age. The most frequent causative agent is respiratory syncytial virus (RSV), although 10%–20% of cases may be caused by other viruses, with coinfection detected in 30% of cases.16,17 Respiratory syncytial virus infects most children within 2 years from birth, but only 20%–30% develop AB and 1%–5% require hospital admission.4,17,18 This percentages increase in children with risk factors, such as preterm birth, congenital heart defects, bronchopulmonary dysplasia or immunodeficiencies.12,17,19 In addition to the classical factors described in the literature, some studies have found that postnatal passive smoking increases the incidence and severity of AB.5,8,11,13,20 However, to date, few prospective studies have assessed the impact of prenatal exposure to tobacco on the development of AB.4,12,21–23
The objective of our study was to establish the incidence of AB and assess the impact of antenatal and postnatal maternal tobacco use in the development of this diseases in the first 2 years of life.
Patients and methodsWe conducted a prospective observational study in a cohort of healthy infants delivered after more than 35 weeks of gestation with a birth weight of more than 2000 g in the maternity ward of the Hospital Clínico Universitario de Valladolid between October 2015 and February 2016. We excluded infants that had neonatal disease and required hospital admission.
The sample was obtained by consecutive sampling, including all mothers willing to participate in the study that completed the self-administered questionnaire that we provided at the time of discharge from the maternity ward for who we obtained data on the use of tobacco during gestation and the daily consumption of cigarettes. We also collected data on sociodemographic, gestational, perinatal and breastfeeding variables. We verified the obstetric and perinatal information provided by the mothers by reviewing the health records.
We followed up the cohort through age 2 years by means of telephone interviews with the parents (mother and/or father) at 6, 12 and 24 months post birth to determine whether the child had received a diagnosis of AB and document the age at diagnosis, the need for hospital admission due to AB and whether the child had experience episodes of wheezing following AB. We defined AB as a first episode of breathing difficulty with wheezing and/or crackles following symptoms of upper respiratory tract infection in a child aged less than 2 years.19 We confirmed the diagnosis of AB by reviewing the electronic health records of the primary care and specialty care systems, which include diagnostic codes of the International Classification of Diseases, Clinical Modification (ICD-9-CM), with code 466.1 corresponding to AB and its categories (466.11 AB due to RSV, 466.19 AB due to other infectious organisms). Given the variability in the diagnosis of this diseases, especially in the second year of life, our definition of AB also included first episodes of symptoms of respiratory infection and breathing difficulty with wheezing and/or crackles coded as acute bronchitis with bronchospasm (ICD-9 code 466.0). We reviewed health care records related to emergency department visits, hospital stays and primary care visits. If we identified inconsistencies between the information given by parents and the information documented in the health records, we used the information provided by the health records in the analysis rather than the information provided by parents.
During the follow-up interviews, we also collected information about tobacco use in both parents, including the number of cigarettes smoked per day, family history of atopy (asthma or allergic rhinitis in parents or siblings), personal history of atopy (atopic dermatitis and food or drug allergies), enrolment in childcare centre, presence of older siblings and duration of breastfeeding (BF) in months.
We corroborated the presence of atopic dermatitis by reviewing the health records in (ICD-9 code 691.8), and disregarded cases that had not been diagnosed by a primary care paediatrician or medical specialist in order to avoid overestimating its incidence. Similarly, we reviewed the records to corroborate the presence of a food or drug allergy, considering diagnosis by a paediatrician conclusive. We collected information on the development of atopic dermatitis and allergies in each telephone interview conducted during the follow-up through age 2 years.
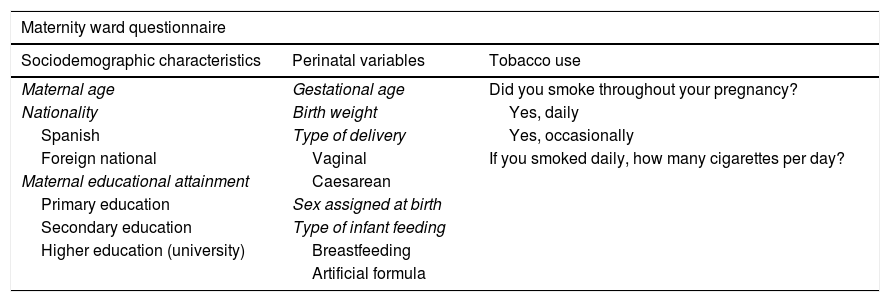
Table 1 presents the questions asked in the maternity ward questionnaire and the follow-up telephone interviews.
Information collected in the questionnaire at discharge from maternity and during the follow-up.
| Maternity ward questionnaire | ||
|---|---|---|
| Sociodemographic characteristics | Perinatal variables | Tobacco use |
| Maternal age | Gestational age | Did you smoke throughout your pregnancy? |
| Nationality | Birth weight | Yes, daily |
| Spanish | Type of delivery | Yes, occasionally |
| Foreign national | Vaginal | If you smoked daily, how many cigarettes per day? |
| Maternal educational attainment | Caesarean | |
| Primary education | Sex assigned at birth | |
| Secondary education | Type of infant feeding | |
| Higher education (university) | Breastfeeding | |
| Artificial formula | ||
| Questions asked in follow-up telephone interviews (6, 12 and 24 months) |
|---|
| Information regarding the diagnosis of acute bronchiolitis |
| Has the child had acute bronchiolitis? |
| Was the child hospitalised due to acute bronchiolitis? |
| Age at onset of acute bronchiolitis (months) |
| Has the child experienced additional episodes of wheezing after the diagnosed episode of acute bronchiolitis? |
| How many episodes of recurrent wheezing has the child experienced post bronchiolitis |
| Family and personal history of atopy |
| Does the mother, father or a sibling have asthma or allergic rhinitis? |
| Does the child have atopic dermatitis? Was it diagnosed by a paediatrician? |
| Does the child have any food or drug allergies? |
| Exposure to tobacco |
| Does the mother smoke? |
| Yes, daily |
| Yes, occasionally |
| If the mother smokes daily: how many cigarettes a day? |
| Does the father smoke? |
| If the father smokes daily: how many cigarettes a day? |
| Other |
| Does the child have older siblings? |
| Is the child currently enrolled childcare? |
| Did the child attend a childcare centre at the time bronchiolitis was diagnosed?a |
| Is the child currently breastfed? |
| If the child is not currently breastfed: how many months was the child breastfed? |
The data were collected by the research team, comprised of paediatricians and medical residents in paediatrics, who administered the questionnaire at discharge from the maternity ward and carried out the telephone interviews during the follow-up.
The study was approved by the Research Committee and Ethics Committee of the Hospital Clínico Universitario de Valladolid and conducted in adherence with the principles of the Declaration of Helsinki.
Statistical analysisThe statistical analysis was performed with the software IBM SPSS 20.0 for Windows® (SPSS Inc., Chicago, Illinois, United States). We summarised categorical variables as percentages and 95% confidence intervals (CIs) and quantitative variables as mean and standard deviation (SD) if the data followed a normal distribution, and otherwise as median and interquartile range (IQR).
We calculated the incidence of AB in the study cohort, the relative risk (RR) of AB and the attributable fraction for prenatal and postnatal exposure to maternal smoking. We performed bivariate and multivariate logistic regression analyses in which the diagnosis of AB was the dependent variable. In the multivariate analysis, we use backward stepwise selection based on the likelihood ratio, and the initial model included the variables with P-values of less than .10 in the bivariate analysis and clinically relevant variables described in previous studies. For the final results of the analysis, we defined statistical significance as a P-value of less than .05. We have presented the results as odds ratios (ORs) with the corresponding 95% CIs.
We calculated the statistical power of the study based on the number of patients with prenatal exposure to tobacco that completed the follow-up.
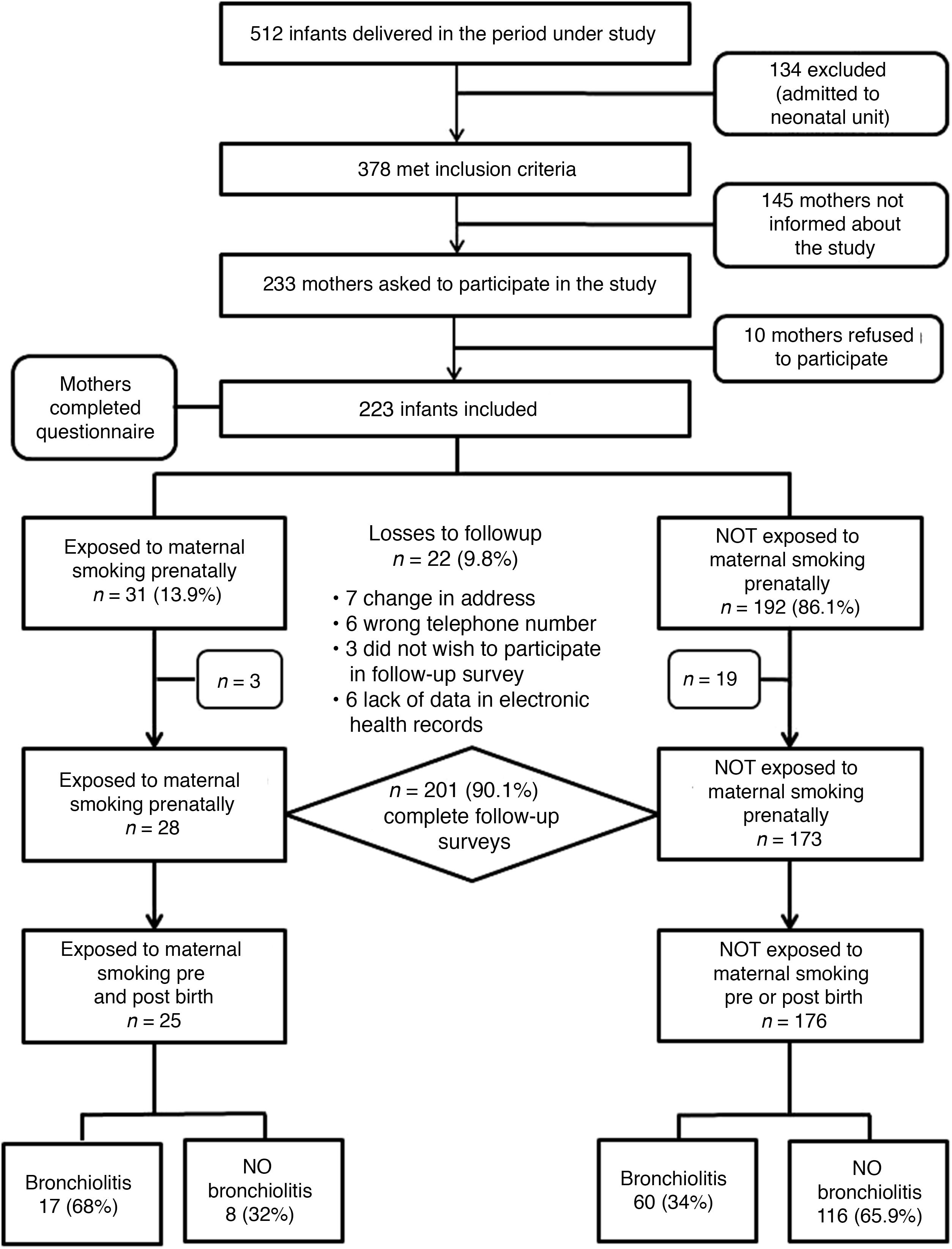
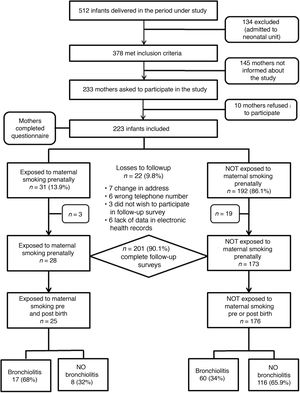
ResultsIn the period under study, a total of 512 infants were managed in our hospital, of who 378 stayed in the maternity ward and met the inclusion criteria. We requested the participation of 233 mothers, of who 10 chose not to complete the questionnaire. Due to a greater volume of patients during holidays and weekends, 145 mothers were not informed about the study or asked to participate.
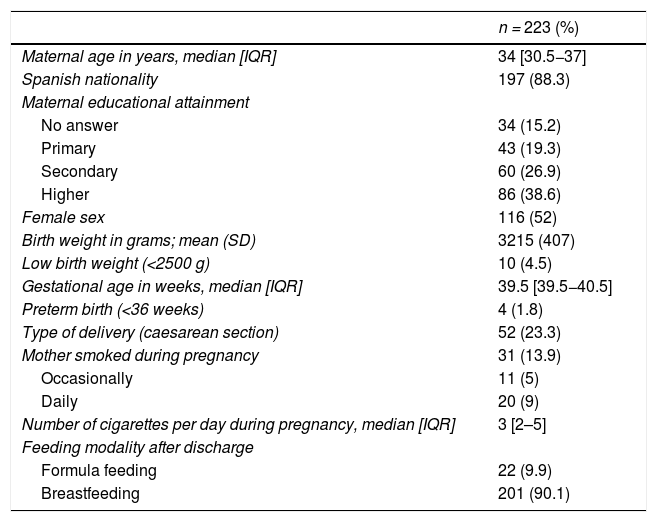
We recruited a total of 223 infants whose mothers completed the questionnaire at the time of discharge from the maternity ward (Fig. 1). Of all mothers, 13.9% (n = 31) smoked during the pregnancy, with a median consumption of 3 cigarettes a day (IQR, 2–5). Table 2 summarises the characteristics of the cohort.
General characteristics of the sample (questionnaire completed at discharge from maternity ward).
| n = 223 (%) | |
|---|---|
| Maternal age in years, median [IQR] | 34 [30.5−37] |
| Spanish nationality | 197 (88.3) |
| Maternal educational attainment | |
| No answer | 34 (15.2) |
| Primary | 43 (19.3) |
| Secondary | 60 (26.9) |
| Higher | 86 (38.6) |
| Female sex | 116 (52) |
| Birth weight in grams; mean (SD) | 3215 (407) |
| Low birth weight (<2500 g) | 10 (4.5) |
| Gestational age in weeks, median [IQR] | 39.5 [39.5−40.5] |
| Preterm birth (<36 weeks) | 4 (1.8) |
| Type of delivery (caesarean section) | 52 (23.3) |
| Mother smoked during pregnancy | 31 (13.9) |
| Occasionally | 11 (5) |
| Daily | 20 (9) |
| Number of cigarettes per day during pregnancy, median [IQR] | 3 [2–5] |
| Feeding modality after discharge | |
| Formula feeding | 22 (9.9) |
| Breastfeeding | 201 (90.1) |
Categorical variables expressed as absolute frequency and percentage, and quantitative variables as mean and standard deviation (SD) or median and interquartile range [IQR].
Seventy-seven patients developed AB, with a cumulative incidence at age 2 years of 34.5% (95% CI, 28.1%–41%); in 64 AB was diagnosed in the first year of life (28.7%; 95% CI, 22.5%–34.9%). The median age at diagnosis was 5 months (IQR, 3.3–11). Ten infants required hospital admission (4.4%) and 47 had recurrent wheezing (21.07%) with a median of 2 episodes of post-bronchiolitis wheezing (IQR, 1–4).
The follow-up through 2 years was completed by 90.1% of patients (n = 201): 28/31 of the patients in the prenatal exposure group and 173/192 of the group without prenatal exposure. The losses were due to changes in address, lack of data in the electronic health records that precluded verification of the information provided in the questionnaires/interviews and withdrawal from the study of parents that chose not to complete the follow-up questionnaires.
In the group of children with prenatal exposure to tobacco, the risk of developing AB was 64.3% (18/28) compared to 34.1% (59/173) in the group without prenatal exposure, with a RR of 1.9 (95% CI, 1.3–2.6) and an attributable fraction of 46.9% (95% CI, 25.1%–62.4%) in the prenatal exposure group.
When it came to the impact of postnatal exposure to tobacco smoke, we found that in the group of 201 participants with a complete follow-up, 43 mothers used tobacco after delivery (21.4%), with a median consumption of 10 cigarettes a day (IQR, 6–10). The risk of AB in the group of children whose mothers smoked after delivery was 53.5% (23/43) compared to 34.2% (54/158) in the group of children whose mothers did not, with a RR of 1.56 (95% CI, 1.1–2.2) and an attributable fraction of 36.1% (95% CI, 9.1–55) in the group exposed to maternal smoking postnatally. We found that 25 children (12.4%) were exposed tobacco in both periods (prenatal and postnatal), with a risk of AB of 68% (17/25) compared to 34% (60/176) in unexposed children, with a RR of 2 (95% CI, 1.42–2.80) and an attributable fraction of 49.9% (95% CI, 29.7–64.3) in children exposed to tobacco before and after birth. Fig. 1 presents a flow chart with the changes in the study cohort and infant outcomes based on the prenatal and postnatal exposure to tobacco.
On the other hand, 66 infants had fathers that smoked (32.8%), with a median consumption of 10 cigarettes a day (IQR, 6–15), 76 had at least one parent who smoked (37.8%) and both parents smoked in 33 cases (16.4%). Table 3 presents the characteristics of patients that completed the follow-up and their distribution based on the diagnosis of AB.
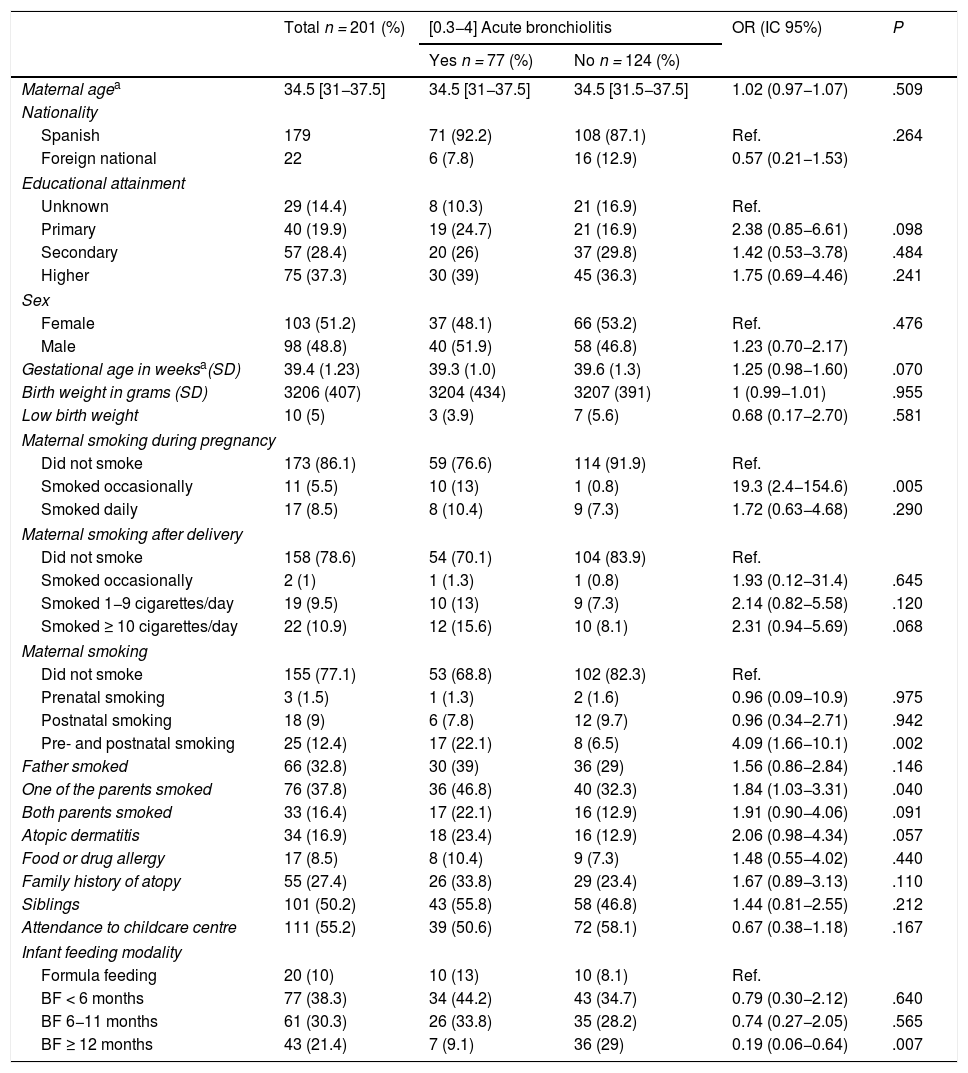
Characteristics of participants that completed the 2-year follow-up and frequency distribution based on the diagnosis of acute bronchiolitis.
| Total n = 201 (%) | [0.3−4] Acute bronchiolitis | OR (IC 95%) | P | ||
|---|---|---|---|---|---|
| Yes n = 77 (%) | No n = 124 (%) | ||||
| Maternal agea | 34.5 [31−37.5] | 34.5 [31−37.5] | 34.5 [31.5−37.5] | 1.02 (0.97−1.07) | .509 |
| Nationality | |||||
| Spanish | 179 | 71 (92.2) | 108 (87.1) | Ref. | .264 |
| Foreign national | 22 | 6 (7.8) | 16 (12.9) | 0.57 (0.21−1.53) | |
| Educational attainment | |||||
| Unknown | 29 (14.4) | 8 (10.3) | 21 (16.9) | Ref. | |
| Primary | 40 (19.9) | 19 (24.7) | 21 (16.9) | 2.38 (0.85−6.61) | .098 |
| Secondary | 57 (28.4) | 20 (26) | 37 (29.8) | 1.42 (0.53−3.78) | .484 |
| Higher | 75 (37.3) | 30 (39) | 45 (36.3) | 1.75 (0.69−4.46) | .241 |
| Sex | |||||
| Female | 103 (51.2) | 37 (48.1) | 66 (53.2) | Ref. | .476 |
| Male | 98 (48.8) | 40 (51.9) | 58 (46.8) | 1.23 (0.70−2.17) | |
| Gestational age in weeksa(SD) | 39.4 (1.23) | 39.3 (1.0) | 39.6 (1.3) | 1.25 (0.98−1.60) | .070 |
| Birth weight in grams (SD) | 3206 (407) | 3204 (434) | 3207 (391) | 1 (0.99−1.01) | .955 |
| Low birth weight | 10 (5) | 3 (3.9) | 7 (5.6) | 0.68 (0.17−2.70) | .581 |
| Maternal smoking during pregnancy | |||||
| Did not smoke | 173 (86.1) | 59 (76.6) | 114 (91.9) | Ref. | |
| Smoked occasionally | 11 (5.5) | 10 (13) | 1 (0.8) | 19.3 (2.4−154.6) | .005 |
| Smoked daily | 17 (8.5) | 8 (10.4) | 9 (7.3) | 1.72 (0.63−4.68) | .290 |
| Maternal smoking after delivery | |||||
| Did not smoke | 158 (78.6) | 54 (70.1) | 104 (83.9) | Ref. | |
| Smoked occasionally | 2 (1) | 1 (1.3) | 1 (0.8) | 1.93 (0.12−31.4) | .645 |
| Smoked 1−9 cigarettes/day | 19 (9.5) | 10 (13) | 9 (7.3) | 2.14 (0.82−5.58) | .120 |
| Smoked ≥ 10 cigarettes/day | 22 (10.9) | 12 (15.6) | 10 (8.1) | 2.31 (0.94−5.69) | .068 |
| Maternal smoking | |||||
| Did not smoke | 155 (77.1) | 53 (68.8) | 102 (82.3) | Ref. | |
| Prenatal smoking | 3 (1.5) | 1 (1.3) | 2 (1.6) | 0.96 (0.09−10.9) | .975 |
| Postnatal smoking | 18 (9) | 6 (7.8) | 12 (9.7) | 0.96 (0.34−2.71) | .942 |
| Pre- and postnatal smoking | 25 (12.4) | 17 (22.1) | 8 (6.5) | 4.09 (1.66−10.1) | .002 |
| Father smoked | 66 (32.8) | 30 (39) | 36 (29) | 1.56 (0.86−2.84) | .146 |
| One of the parents smoked | 76 (37.8) | 36 (46.8) | 40 (32.3) | 1.84 (1.03−3.31) | .040 |
| Both parents smoked | 33 (16.4) | 17 (22.1) | 16 (12.9) | 1.91 (0.90−4.06) | .091 |
| Atopic dermatitis | 34 (16.9) | 18 (23.4) | 16 (12.9) | 2.06 (0.98−4.34) | .057 |
| Food or drug allergy | 17 (8.5) | 8 (10.4) | 9 (7.3) | 1.48 (0.55−4.02) | .440 |
| Family history of atopy | 55 (27.4) | 26 (33.8) | 29 (23.4) | 1.67 (0.89−3.13) | .110 |
| Siblings | 101 (50.2) | 43 (55.8) | 58 (46.8) | 1.44 (0.81−2.55) | .212 |
| Attendance to childcare centre | 111 (55.2) | 39 (50.6) | 72 (58.1) | 0.67 (0.38−1.18) | .167 |
| Infant feeding modality | |||||
| Formula feeding | 20 (10) | 10 (13) | 10 (8.1) | Ref. | |
| BF < 6 months | 77 (38.3) | 34 (44.2) | 43 (34.7) | 0.79 (0.30−2.12) | .640 |
| BF 6−11 months | 61 (30.3) | 26 (33.8) | 35 (28.2) | 0.74 (0.27−2.05) | .565 |
| BF ≥ 12 months | 43 (21.4) | 7 (9.1) | 36 (29) | 0.19 (0.06−0.64) | .007 |
BF, breastfeeding; CI, confidence interval; OR, odds ratio; Ref, reference group.
Categorical variables expressed as absolute frequency and percentage, and quantitative variables as mean and standard deviation (SD) or median and interquartile range [IQR].
We assessed the duration of BF in the groups of mothers that smoked and mothers that did not smoke after delivery and found a mean duration of 4 months (95% CI, 2.8–6.2) in the group of mothers that smoked compared to 6 months (95% CI, 6.6–8.6) in the non-smoking group.
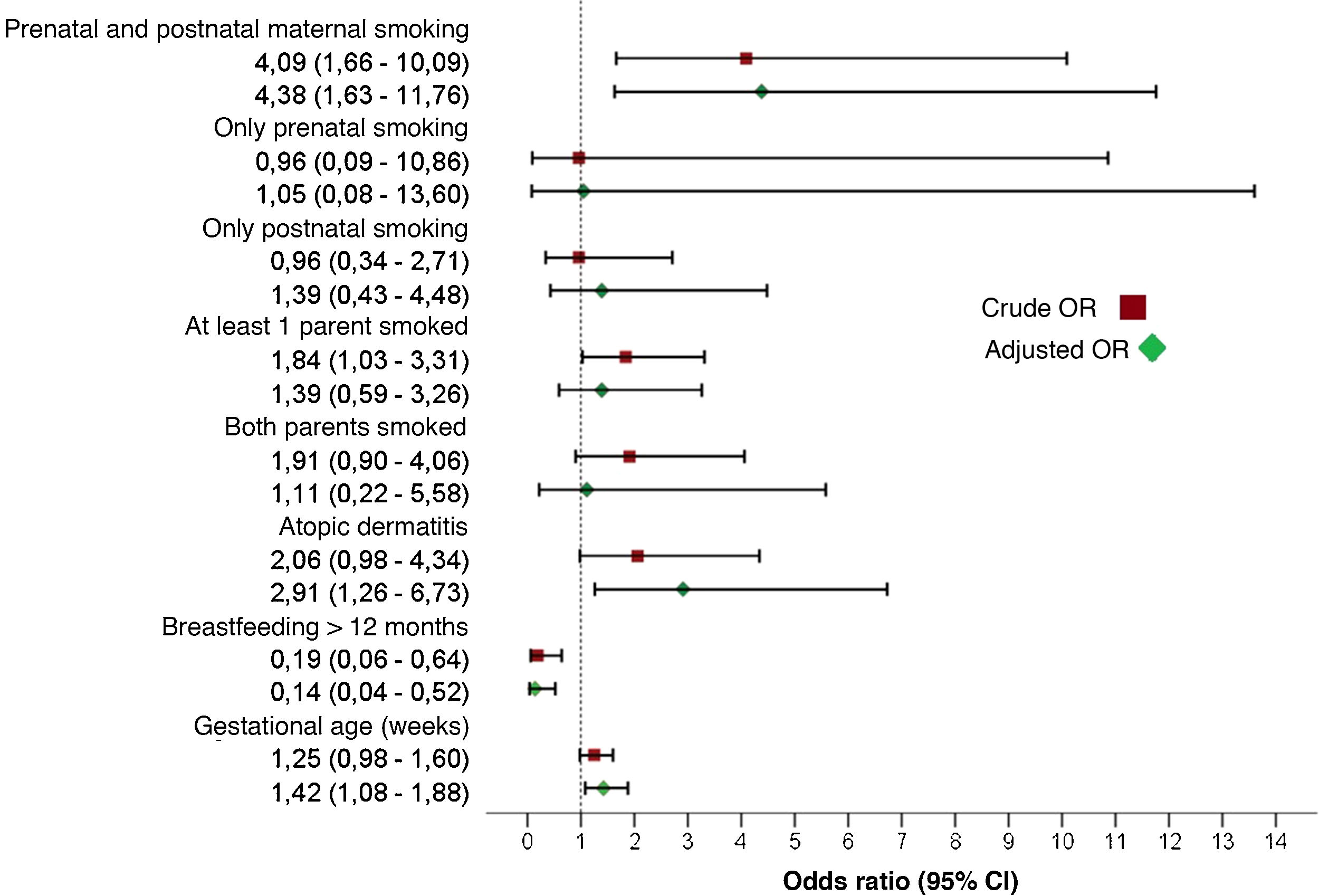
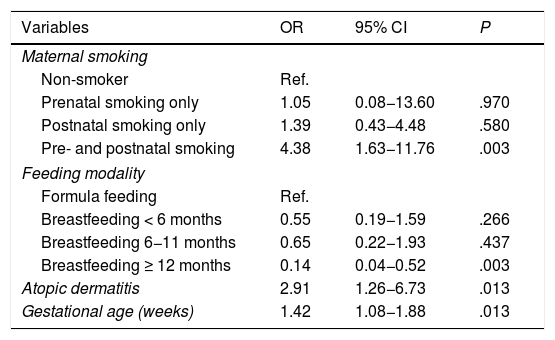
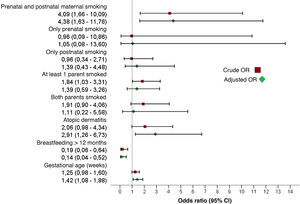
Bivariate and multivariate analysesTable 3 presents the results of the bivariate analysis. The multivariate analysis identified the independent risk factors for development of AB (Table 4). We observed that prenatal and postnatal maternal smoking corresponded to a 4.38-fold increase in the risk of AB (95% CI, 1,63–11.76). Other factors with a statistically significant association with AB were a history of atopic dermatitis (OR, 2.91; 95% CI, 1.26−6.73), gestational age at birth (OR, 1.42; 95% CI, 1.08−1.88) and duration of BF of 12 months or greater, which exhibited a protective effect (OR, 0.14; 95% CI, 0.04−0.52). Fig. 2 summarises the effect on the development of AB of prenatal and postnatal maternal smoking and of the variables included in the multivariate model.
Risk factors for acute bronchiolitis in the first 2 years of life. Multivariate analysis (Nagelkerke R2, 0.21).
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Maternal smoking | |||
| Non-smoker | Ref. | ||
| Prenatal smoking only | 1.05 | 0.08−13.60 | .970 |
| Postnatal smoking only | 1.39 | 0.43−4.48 | .580 |
| Pre- and postnatal smoking | 4.38 | 1.63−11.76 | .003 |
| Feeding modality | |||
| Formula feeding | Ref. | ||
| Breastfeeding < 6 months | 0.55 | 0.19−1.59 | .266 |
| Breastfeeding 6−11 months | 0.65 | 0.22−1.93 | .437 |
| Breastfeeding ≥ 12 months | 0.14 | 0.04−0.52 | .003 |
| Atopic dermatitis | 2.91 | 1.26−6.73 | .013 |
| Gestational age (weeks) | 1.42 | 1.08−1.88 | .013 |
CI, confidence interval; OR, odds ratio; Ref, reference group.
Variables included in the analysis: maternal smoking, infant feeding modality, 1 smoking parent, 2 smoking parents, atopic dermatitis, gestational age (weeks).
Effect of prenatal and postnatal maternal smoking on the development of acute bronchiolitis. The combination of both prenatal and postnatal smoking was a statistically significant risk factor for developing acute bronchiolitis, while separately neither factor was statistically significant. The figure shows the crude and adjusted odds ratios of the variables included in the multivariate model.
Assuming a prevalence of prenatal exposure to tobacco through maternal smoking of 13.9%, a ratio of not exposed/exposed (r) of 6.2 and a risk of AB of 64.3% in children exposed to tobacco before birth and 34.1% in children not exposed to tobacco prenatally, the statistical power was 85%.
DiscussionThe data obtained in our study show a cumulative incidence of AB of 28.7% at 1 year and 34.5% at 2 years post birth, similar to the figures reported in other case series.24,25 The main finding of the study was that there was a significant increase in the risk of AB associated with prenatal and postnatal exposure to tobacco, which was an independent risk factor for the development of AB in the first 2 years of life.
The reported incidence of AB varies between published studies, probably due to the heterogeneity of diagnosis in different countries. While in the United States expiratory wheezing is considered the main feature for diagnosis, in the United Kingdom it is crackles.19 In Spain, the McConnochie criteria is the most widely accepted approach to diagnosis, to be made in children with a first episode of respiratory distress with wheezing of acute onset preceded by manifestations compatible with viral respiratory illness before age 2 years (most frequently before 1 year).18 This broad clinical spectrum entails a degree of variability in the incidence reported by different studies. In a prospective study conducted by Verhulst et al.25 in a region of Belgium that followed up 154 full-term infants, 23.5% had at least one episode of wheezing in the first 12 months of life. In Spain, data from the Asthma Multicenter International Cohort Study (AMICS) show a cumulative incidence of wheezing in the first year of life of 28% in Barcelona and 24% in Menorca.24 The published data on the incidence of AB in the first 2 years of life are scarce, so we consider that our study makes a relevant contribution by providing updated data for our population.
As we already mentioned, 13.9% of mothers reported having smoked during pregnancy. The prevalence of maternal smoking during pregnancy reported in our study was similar to the prevalence reported in other studies conducted in Spain.26,27
The results of our study demonstrate the effects of maternal smoking in the development of AB. We found that children of mothers that smoked during and after pregnancy were twice as likely to develop AB in the first 2 years of life compared to children of non-smoking mothers. Based on the attributable fraction in the group of children exposed to tobacco in both periods (prenatal and postnatal), 49.9% of episodes of AB in children of smoking mothers can be attributed to this risk factor.
There is a growing interest about the potential deleterious impact of intrauterine exposure to tobacco on foetal pulmonary development.12,18,26–31 There is evidence that children of mothers that smoked during pregnancy have abnormal results in pulmonary function tests in early childhood that may persist through adulthood, with frequent obstruction of medium- and small-calibre airways.6,32–34 Animal models of the effects of nicotine on pulmonary development have found that exposure to nicotine in pregnant dams causes antenatal changes in airway development, with surprising interspecies similarities, including increased collagen deposition under the basement membrane, increased expression of the MUC5AC gene (secretory mucin glycoprotein mucin-5AC) in the bronchial epithelium, increased thickness of smooth muscle and decreased airway calibre.35 All these changes could account for the sequelae that tobacco exposure can cause in foetal lung development in humans and the subsequent predisposition to develop wheezing episodes.
Some authors have proposed a transgenerational influence of prenatal tobacco smoke exposure on the development of wheezing and asthma. The Norwegian Mother and Child Cohort Study found that tobacco use in grandmothers was associated with an increased risk of asthma in grandchildren at ages 3 and 7 years, independently of maternal smoking during pregnancy. The authors of this study hypothesised that transgenerational epigenetic changes associated with DNA methylation could explain this finding.36 In our study, we did not collect data on the smoking habits of grandmothers, although this would be an interesting variable to analyse in future studies.
Different studies have demonstrated that postnatal exposure to tobacco is a risk factor for lower respiratory tract infection in the early years of life.5,9,11,23 Exposure to tobacco smoke alters the defence mechanisms of the respiratory tract, such as mucociliary clearance, and impairs the innate immune response, thereby increasing susceptibility to respiratory infection by different pathogens.4 Since most of the women that smoke during pregnancy continue to smoke after delivery, it is difficult to determine whether the adverse effect of tobacco is due to the impact on foetal development during gestation or to postnatal exposure. The findings of previous studies on the subject have been contradictory. Cano-Fernández et al.12 analysed the effect of prenatal and postnatal exposure to tobacco on the development of AB and found that only maternal smoking during gestation was an independent risk factor for AB in the multivariate analysis (OR, 3.27; 95% CI, 1.39–7.71). On the other hand, Behrooz et al.23 found that prenatal exposure to tobacco was not a significant risk factor for bronchiolitis, while postnatal exposure to smoke was associated with a 4-fold increase in the risk of AB in the analysis adjusted for other variables (OR, 4.19; 95% CI, 2.51–6.98). Neither study analysed the impact of exposure to tobacco in both periods, as we did in our study. The changes in the immune response and defence mechanisms of the airway caused by postnatal exposure to tobacco probably compound the damage that prenatal exposure caused in the developing airway, increasing the risk of lower respiratory tract infection and wheezing.
We found that 37.8% of children were exposed to second-hand smoke, as at least one of their parents smoked (mother, father or both), although this factor, possibly due to the greater effect of maternal smoking on the development of AB, was not significantly associated with AB in the multivariate analysis.
Previous studies have demonstrated that maternal smoking affects children more than paternal smoking,7 probably due to the closer contact of mothers with their children.
Among the other factors under study, we ought to highlight that a higher proportion of patients with AB had atopic dermatitis in the first 2 years of life. Although atopic dermatitis is a known risk factor for asthma,37 it has not been studied thoroughly in patients with AB.38 A prospective study in a cohort of pregnant women with follow-up of their children found a significant association between atopic dermatitis and severe AB in the first year of life (OR, 2.72; 95% CI, 1.60–4.63).38 Although the mechanisms underlying this association are not understood, the authors proposed that changes in the epithelial barrier may play a significant role. Variants in the cadherin family (glycoproteins involved in cell adhesion) have been associated with eczema, bronchial hyperresponsiveness and asthma, and more recently cadherin 3 (CDHR3) has been identified as a receptor for rhinovirus, one of the causative agents of AB.39 As was the case in our cohort study, the analysis of these authors did not allow the establishment of causal relationships, but these results are interesting nevertheless, as they could guide future research on the clinical significance of this association in the development of asthma.
We found that BF lasting more than 12 months had a protective effect against the development of AB. We also found that maternal smoking was negatively associated with the duration of BF, which was shorter in smoking mothers, which could indicate that BF could act as a confounder through an association with smoking status. However, the multivariate analysis corroborated the beneficial effect of extended BF on the development of AB in our cohort, independently of maternal smoking status. The benefits of BF in the prevention of infectious diseases in early childhood are well known, and their protective effect in relation to AB, even in children exposed to second-hand smoke, has been demonstrated by previous studies.13,24,25,40
We believe that the significant association between greater gestational age and AB was a spurious finding. Since our cohort only included infants born at term or near term, gestational age had little relevance in our analysis.
Some of the variables identified in the previous literature as risk factors for AB were not significant in our study. While we found a subtle positive association with male sex, low maternal educational attainment and having an older sibling, the association was not statistically significant. In contrast to the findings of other studies, we did not find an association between enrolment in a childcare centre and AB. We ought to underscore that our cohort consisted of healthy infants born at term of near term and with a low proportion of low birth weight. Therefore, we were able to study the impact of prenatal and postnatal exposure to tobacco while excluding other factors that have been investigated thoroughly in the past.
In agreement with previous case series, most of the children in our cohort that developed AB had mild symptoms and did not require hospital admission. Due to the small number of patients that developed severe AB, we were not able to analyse the effect of maternal smoking on the need of hospital admission.
One of the strengths of our study is its prospective design with follow-up through age 2 years, which provided data on the incidence of AB in our region in the first 24 months of life, a period for which the published data are scarce. In addition, the losses during the follow-up were small (9.8%) and the achieved statistical power was sufficient to support the validity of our findings. Among the limitations, we ought to mention that the data were collected by means of questionnaires. The information provided by mothers at discharge from the maternity ward was collected through a self-administered questionnaire, which resulted in a varying percentage of mothers not answering certain items, such as the maternal educational attainment, possibly because the respondents considered them an invasion of their privacy. This could have been a source of response bias, although we collected the data on other demographic, obstetric and perinatal variables from the health records. Unexpectedly, 100% of respondents answered the question about tobacco use during pregnancy, although we think there is still a risk of information bias due to the possible underreporting of tobacco use by mothers. We obtained information about the diagnosis of AB from telephone interviews; however, we verified this information by reviewing the electronic health records, counting only those cases that met the established criteria. Similarly, we obtained information on passive smoking by the parents, and we do not know the reason why some of the parents refused to complete the follow-up. Given the social stigma attached to parental smoking, the data on postnatal tobacco exposure collected in our study may have led to underestimation of the prevalence of passive smoking in children, as there was a risk of information bias. To improve the accuracy of the assessment of passive smoking in children, it would be interesting to use methods such as the measurement of cotinine, which is not only more objective but also allows quantification of the exposure.
ConclusionsChildren of smoking mothers are at increased risk of AB from birth. Prenatal and postnatal exposure to tobacco are preventable risk factors, and therefore decreasing tobacco use in women seeking to conceive should be a priority of preventive medicine. Effective strategies must be developed to help prevent prenatal and postnatal exposure to tobacco, as well as interventions to promote initiation and maintenance of BF given the protective effect of BF on the development of AB and other infectious diseases of childhood.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Carmen Fernández García-Abril for developing the database for the study, Sergio Madero Juez for his contributions to the revision of the manuscript and María Fe Muñoz Moreno for her collaboration in the statistical analysis.
Please cite this article as: Bermúdez Barrezueta L, Miñambres Rodríguez M, Palomares Cardador M, Torres Ballester I, López Casillas P, Moreno Carrasco J, et al. Efecto de la exposición prenatal y postnatal al tabaco en el desarrollo de bronquiolitis aguda durante los dos primeros años de vida. An Pediatr (Barc). 2021;94:385–395.