It has been suggested that neuromuscular blockade (NMB) affects the capacity of bispectral index (BIS) monitoring to measure consciousness in sedated children. Our aim was to analyse the impact of NMB on BIS values in critically ill children.
MethodsWe conducted a prospective observational study of children monitored with a BIS system that received a continuous infusion of vecuronium. We analysed data on clinical, diagnostic and haemodynamic variables, sedatives, analgesics, muscle relaxants, and BIS parameters. We compared BIS parameters before the use of a muscle relaxant, during its administration, before its discontinuation and for the 24h following the end of the infusion.
ResultsThe analysis included 35 patients (median age, 30 months). The most common diagnosis was heart disease (85%). The most frequent indication for initiation of NMB was low cardiac output (45%), followed by adaptation to mechanical ventilation (20%). Neuromuscular blockade did not produce a significant change in BIS values. We found a decrease was observed in electromyography (EMG) values at 6h (34.9±9.4 vs 31.2±7; P=.008) and 12h after initiation of NMB (34.9±9.4 vs 28.6±4.8; P =.006). We observed a small significant increase in BIS after discontinuation of NMB (from 42.7±11 to 48.4±14.5, P=.001), and 6 and 12h later (51.3±16.6; P=.015). There were no differences in the doses of sedatives or analgesics except for fentanyl, of which the dose was lowered after discontinuation of vecuronium.
ConclusionContinuous NMB produces small changes on BIS values that are not clinically significant and therefore does not interfere with BIS consciousness monitoring in critically ill children.
Estudios previos sugieren que el bloqueo neuromuscular (BNM) altera la monitorización del índice bispectral (BIS) en los niños sedados. El objetivo fue analizar la repercusión del uso y suspensión del BNM en la monitorización BIS en niños críticamente enfermos.
MétodosEstudio observacional prospectivo. Se incluyeron los niños que recibían perfusiones intravenosas de vecuronio con monitorización BIS. Se analizaron variables clínicas, diagnósticas, hemodinámicas, sedoanalgesia y relajantes musculares y parámetros del BIS. Se compararon los valores del BIS antes del uso de relajantes neuromusculares (RNM), durante su administración, antes de su retirada y durante las 24h siguientes a su suspensión.
ResultadosTreinta y cinco pacientes (edad mediana 30 meses). El diagnóstico más frecuente fue cardiopatía (85%). Las indicaciones más frecuentes para iniciar RNM fueron bajo gasto cardiaco (45%) y adaptación a ventilación mecánica (20%). El BNM no produjo cambios significativos en los valores del BIS. Se observó una disminución de los valores del electromiograma a las 6h (34.9±9.4 vs 31.2±7; p=0.008) y a las 12h del inicio de la perfusión de vecuronio (34.9±9.4 vs 28.6±4.8; p=0.006). Tras retirar el vecuronio hubo un ligero aumento significativo del BIS (de 42.7±11 a 48.4±14.5, p=0.001), así como en las siguientes 6 y 12h (51.3±16.6; p=0.015). No hubo diferencias en las dosis de sedantes o analgésicos, excepto del fentanilo, que fue disminuido tras retirar el vecuronio.
ConclusiónEl BNM continuo produce pequeños cambios en los valores del BIS sin relevanciaclínica, y no altera la monitorización del nivel de conciencia del BIS en los niños críticamenteenfermos.
The bispectral index (BIS) estimates the level of brain activity through a mathematical analysis of the frequencies of waves in electroencephalography (EEG) signals and it also obtains information from electromyography (EMG) data.
The BIS index was first introduced in 1992 by Aspect Medical Systems. The main component of the BIS monitor is the bispectral analysis, which evaluates the phase relations from a single channel EEG signal measured from the patient’s forehead. The BIS index is a dimensionless number from 0 to 100. Firstly, the EEG signal is digitized and pre-processed. BIS includes two types of burst suppression (BS) detection: the burst-suppression ratio (BSR) and the QUAZI-Suppression. The pre-processed data is used to calculate the β-ratio parameter (a ratio of empirically measured frequency bands, 30–47Hz and 11–20Hz). Simultaneously, the second parameter, synch-fast-slow, is calculated from bispectral analysis. Finally, all parameters are then fed to the weighting algorithm, which produces the BIS index.1 Electromyographic data corresponds to the 30−50Hz range, which can overlap with BIS.
BIS was initially used for the objective assessment of sedation depth in patients undergoing general anaesthesia, but it has subsequently proved to be useful in monitoring the level of sedation in patients admitted to the ICU,2–4 especially those under deep sedation.5 International guidelines recommend its use for monitoring patients treated with muscle relaxants in who it is not possible to use clinical scores.6,7
Neuromuscular blockade (NMB) decreases electromyographic activity. Some authors have suggested that the administration of these drugs may also decrease BIS values and overestimate the degree of patient´s sedation.8 Several studies, most of them conducted in adult patients, have analysed the impact of NMB on BIS, with contradictory results.9–14 Many have found that neuromuscular blocking agents (NMBAs) decrease the EMG component of BIS, thus reducing the value of the latter.8,10,14 However, some authors attribute the impact of NMB on BIS to a coincidental deeper level of sedation in those cases.10,12
Our hypothesis was that NMB does not alter the ability of BIS to monitor consciousness in sedated children in paediatric intensive care units (PICUs).
The aim of our study was to analyse the impact of the use or discontinuation of NMB on BIS values in critically ill children.
Patients and methodsWe conducted a prospective observational study in a tertiary referral hospital in Madrid, Spain, between 2011 and 2012. We included all patients aged 1 month to 16 years admitted to PICU during the period under study that were monitored with BIS and required infusion of NMBAs. We excluded patients with a duration of NMB of less than 6h and those with seizures or encephalopathy that could affect BIS values. The study was approved by the local ethics committee.
We collected data on demographic (age, sex) and clinical characteristics (reason for admission, underlying disease), haemodynamic parameters (blood pressure, heart rate, central venous pressure and urine output), inotropic therapy, mechanical ventilation and reason for using NMB. Sedatives and analgesic drugs were administered per the PICU protocol, which adheres to the guidelines of the Sociedad Española de Cuidados Intensivos Pediátricos (Spanish Society of Paediatric Intensive Care). The protocol includes the rotation of different sedative and analgesic drugs with the aim of minimising withdrawal symptoms on discontinuation of opioids and benzodiazepines. We recorded the administered sedatives and analgesics and their dosage. The NMBA used in every case was vecuronium, always given at a rate of 0.1mg kg/h.
Bispectral index monitoring was performed with a BIS XP Aspect Medical Systems® monitor and paediatric BIS sensors. Bispectral index values were recorded continuously, as were values of electromyographic (EMG) parameters, the signal quality index (SQI), the total power (TP) and the spectral edge frequency (SEF). We measured and recorded BIS parameters, the blood pressure (BP) and the heart rate (HR) 30min before initiation of NMB and 6, 12 and 24h thereafter. We also measured and documented the same variables were also before discontinuing BNM, and 6, 12 and 24h after discontinuation. At the same time, we monitored the level of pain and of sedation using internationally validated scales such as the COMFORT behaviour scale and the pain ladder, and adjusted the infusion of sedatives and analgesics based on the resulting scores.
We defined moderate sedation as a BIS value of 40–60 and deep sedation as a BIS value of less than 40.15 We discarded BIS values when the signal quality index was less than 60% or the impedance level was greater than 10kΩ.
Statistical analysisWe analysed the data using the software SPSS Statistics version 19. We used the Fisher exact test to compare frequencies and the Mann-Whitney U test to compare quantitative variables. Also, we used the Wilcoxon test to compare BIS, EMG, SQI, BP and HR values before and after NMB. We analysed the correlation between nonparametric variables by means of the Spearman rank correlation coefficient. We defined statistical significance as a p-value of less than 0.05.
ResultsThe analysis included 35 patients with a mean age of 30 months (range, 2–288 months), 52.9% male. The primary diagnosis in 85% of cases was postoperative care after cardiac surgery, followed by head injury (9%) and bronchiolitis (6%). The most frequent reasons for use of NMB administration were haemodynamic instability (low cardiac output, ECMO, junctional ectopic tachycardia; 49%), improvement of patient-ventilator synchrony (23%), pulmonary hypertension (14%) and increased intracranial pressure (6%).
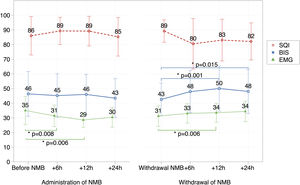
Fig. 1 presents the BIS, EMG and SQI values. The SQI was above 80% at all times. Before administration of NMBAs, patients were under moderate-to-deep sedation (mean BIS, 46.3±14.9). The mean EMG value before starting the NMB infusion was 34.9±9.4.
Evolution of BIS, EMG and SQI values.
BIS, bispectral index; EMG, electromyography; SQI, signal quality index; NMB, neuromuscular blocker *: statistically significant.
BIS values: Comparison between BIS values at different time points: prior to NMB infusion and after 6h of infusion (P=.141); before NMB and at 12h of infusion (P=.344); before NMB and at 24h of infusion (P=.42); discontinuation of NMB and 6h later (P=.001); discontinuation of NMB and 12h later (P=.015).
EMG values: Comparison between EMG values at different time points: before NMB and at 6h of infusion (P=.008); before NMB and at 12h of infusion (P=.006); discontinuation of NMB and 6h later (P=.08); discontinuation of NMB withdrawal and 12h later: (P=.012).
We did not observe significant changes on BIS values with the administration of vecuronium infusion (Fig. 1). However, we found a small but significant increase in BIS values 6h (P=.001) and 12h after discontinuing the NMBA (P=.015).
We observed a decrease in electromyogram (EMG) values at 6h (34.9±9.4 vs 31.2±7; P=.008) and 12h after initiation of NMB (34.9±9.4 vs 28.6±4.8; P = .006). There was a small significant increase in BIS on discontinuation of NMB (from 42.7±11 to 48.4±14.5; P=.001), as well as 6 and 12h after discontinuation (51.3±16.6; P=.015). Furthermore, EMG values increased significantly after discontinuation of NMB at 6h (P=.08) and 12h (P=.012). We did not find any other statistically significant changes in BIS parameters.
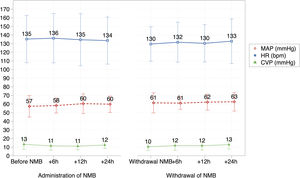
We did not find a significant correlation between BIS and EMG values before and after NMB. We also found no significant changes in haemodynamic parameters during the infusion or after discontinuation of NMB (Fig. 2).
There were no differences in the sedatives and analgesics used during these periods or their doses, save for the dose of fentanyl, which was significantly reduced after discontinuation of NMB (at 6h, 2.2±1.6 vs 1.9±1.6; P = .011 and at 12h, 2.2±1.6 vs 1.7±1.6; P = .01) (Fig. 3).
Changes in sedatives and analgesics.
NMB, Neuromuscular blocker.
There were no differences in the doses of sedatives or analgesics, except for the dose of fentanyl, which was significantly reduced after discontinuation of the NMB infusion (at 6h, 2.2±1.6 vs 1.9±1.6 [P=.011]; at 12h, 2.2±1.6 vs 1.7±1.6 [P=.01]).
Our study shows that the administration of NMBAs in children does not alter BIS significantly, so the latter is a useful method for monitoring sedation in patients under NMB.
Bruhn observed that, after administering NMB to a volunteer, electromyographic activity could distort BIS measurements,9 and other research groups have since confirmed these findings.8,13–16 However, other studies, most of them conducted in adults,8–16,18–20 have not found significant changes in those measurements.10,12,17,18
Weber et al. compared the effect on BIS values of a dose of muscle relaxant versus placebo after anaesthetic induction in 40 children that were undergoing surgery. They found no differences between the treatment and the placebo groups.17
We conducted our study in critically ill children. Rather than analysing the effect on BIS values of a single dose of NMBA, we studied the effect of continuous infusion of NMB and its discontinuation. Our results showed no relevant changes in BIS values with administration of NMB, although we did find a small but significant increase in BIS values after discontinuation of vecuronium. However, although there was a statistically significant change in BIS values, they remained within the 40-to-60 range (moderate sedation).
Previous studies that showed that BIS was affected by NMBAs were mostly performed in patients under moderate sedation or in conscious volunteers.13 However, studies conducted in patients under deep sedation have not found significant changes in BIS values.21
This could explain the discrepancies between the findings of different studies, as EMG activity probably affects BIS values in conscious patients. When NMB is used, EMG activity decreases and causes a secondary drop in BIS values.10 In contrast, our patients were already under moderate or deep sedation with low EMG activity before the initiation of NMB. This probably explains why the infusion of the NMBA was associated with a decrease in EMG values and NMB discontinuation with minor increase in EMG values, which did not have a significant effect on the BIS.
In agreement with the findings of other authors,8,10,12,14 we observed statically significant changes on BIS and EMG values 6 and 12h after discontinuing the NMB infusion. These changes may be explained in part by muscular contraction, but they are so small that we think they do not have any clinical significance in the assessment of the level of sedation.
In addition, we found no correlation between BIS values and haemodynamic parameters (blood pressure and heart rate), which was consistent with previous studies.22 A probable explanation is that these parameters are influenced by many different factors, and not only the degree of sedation or relaxation of the patient.
There are several limitations to our study. The sample size was relatively small, which could reduce the statistical validity of comparisons. In addition, all of the participants were under deep sedation because, for ethical reasons, we do not use muscle relaxation in patients with mild sedation. Furthermore, our study did not measure the degree of muscle relaxation.8,10,14
ConclusionContinuous infusion of NMB produces small changes on BIS values in critically ill children under moderate-to-deep sedation that are not clinically relevant, and therefore it does not compromise BIS monitoring of the level of consciousness in critically ill children.
We thank our colleagues at the PICU of the Hospital Gregorio Marañón, who volunteered to participate in our study and contributed expertise and insights that were very helpful in the research project.
Please cite this article as: Sanavia E, et al. Efecto del bloqueo neuromuscular sobre la monitorización biespectral en los niños críticamente enfermos. An Pediatr (Barc). 2020;93:251–256.





![Changes in sedatives and analgesics. NMB, Neuromuscular blocker. There were no differences in the doses of sedatives or analgesics, except for the dose of fentanyl, which was significantly reduced after discontinuation of the NMB infusion (at 6h, 2.2±1.6 vs 1.9±1.6 [P=.011]; at 12h, 2.2±1.6 vs 1.7±1.6 [P=.01]). Changes in sedatives and analgesics. NMB, Neuromuscular blocker. There were no differences in the doses of sedatives or analgesics, except for the dose of fentanyl, which was significantly reduced after discontinuation of the NMB infusion (at 6h, 2.2±1.6 vs 1.9±1.6 [P=.011]; at 12h, 2.2±1.6 vs 1.7±1.6 [P=.01]).](https://static.elsevier.es/multimedia/23412879/0000009300000004/v2_202106040604/S2341287920300077/v2_202106040604/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95erwEulN6Tmh1xJpRhO+VE=)


