In December 2013, in a remote area of Guinea, perhaps through direct contact with bats carrying the Ebola virus, a child fell ill and died a few days later.1 Starting, possibly, from this case, the disease gradually spread, first among his family members and carers, then among their contacts, and finally travelling with ease through highly permeable border areas, at first among small population centres in Guinea-Conakry, Liberia and Sierra Leone, and subsequently reaching the capitals of these countries.
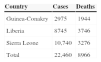
The epidemic did not go unnoticed, but its importance was clearly underestimated and it did not receive the appropriate response at the right moment, that is, at the start. A year later this is undoubtedly the worst epidemic in the history of Ebola virus disease (EVD), with over 20,000 recorded cases by the end of 2014 and nearly 8000 fatalities (Table 1), including 800 cases among health care workers. The charity Médecins Sans Frontières (MSF) raised the alarm as early as Spring 2014, warning that “the epidemic was out of control”. The WHO finally declared the situation a “Public Health Emergency of International Concern” in August 2014, following the guidelines of the International Health Regulations.2–4
The epidemic also produced isolated outbreaks in 2014, originating from imported cases, in Nigeria, Senegal and Mali. More than two dozen infected and sick expatriates (health care workers) have been evacuated to various countries in the European Union and to the United States for treatment. Two imported cases of EVD were recorded in the USA and three secondary cases among health care staff treating patients with EVD (two nurses in Dallas and a nursing assistant in Madrid), and it was then that Western public opinion and media devoted the greatest attention to the epidemic, which came to be described as a “threat to national security”. Soon after this the United Nations set up a special committee (UNMEER: the UN Mission for Ebola Emergency Response) to coordinate the international response, led basically by the USA, the United Kingdom and France themselves, with aid focused respectively on Liberia, Sierra Leone and Guinea-Conakry; this aid is still proving insufficient and is (and has been) arriving several months late. So far Spain has hardly contributed to this international response.5
The differential causes of this epidemic must be sought in the geography, demography and social situation of the affected area. West Africa had not previously suffered outbreaks of EVD, although in some parts of the region there have been small and sporadic outbreaks of Lassa fever. This natural reservoir of the virus in West Africa was probably introduced a few years ago by the migration of the species of bats that are potential carriers of the Zaire subtype of Ebola virus (EBOV), which is responsible for the current epidemic.
The three capitals of the affected countries, Monrovia (Liberia), with a population of 1.1 million, Freetown (Sierra Leone), with a population of 1 million, and Conakry (Guinea-Conakry), with a population of 2.2 million, have features in common that are significant for understanding the situation. They are the largest cities in each country, they have very active commercial ports, they have international airports and the average resident of these cities lives on an income of less than US $2 per day (the poverty line). Moreover, all the affected countries have very weak health care systems. Life expectancy at birth in 2012 was 62 years in Liberia, 58 in Guinea-Conakry and 46 in Sierra Leone (in Spain it is 80). The figures for under-5 mortality are horrifying: 75 per 1000 live births in Liberia, 100 per 1000 live births in Guinea-Conakry and 182 per 1000 live births in Sierra Leone (in Spain it is 4 per 1000 live births). This merely reflects the dreadful situation that already existed in the region, in terms of poverty and poor health care, before the EVD epidemic. Annual per capita health care spending is US $65 in Liberia, $32 in Guinea-Conakry and $96 in Sierra Leone (in Spain it is over $3000). Health care staff are very scarce: Liberia has 1 doctor per 100,000 inhabitants, Guinea-Conakry 10 per 100,000 and Sierra Leone 2 per 100,000 (in Spain there are 370 doctors per 100,000 inhabitants). In addition, the population of these countries do not have much confidence in their governments (after decades of civil war and corruption) and there are not only numerous religious and cultural beliefs, but also rumours, which have hampered the provision of health aid or the application of control measures that have proved effective in other EVD epidemics. These measures are rapid identification of cases, isolation and treatment in centres equipped to ensure hygiene and prevent transmission of the infection, follow-up of all contacts and safe burial of infected patients that have died.5,6
The clinical description of cases can be consulted in recent articles.7,8 The strain of the virus is similar to that which has caused other epidemics, the mean incubation period (generally 2–21 days) is about 8–9 days, its mechanism of transmission is the same (direct contact with blood and body fluids from patients with symptoms, basically in the final stages of the disease), its basic reproduction number (R0) is between 1 and 2 (so it is not very contagious), the clinical symptoms are similar (a notable point is that haemorrhaging is present in only 18% of cases) and the duration of the disease (5–7 days) is also similar. The recorded fatality rate is 50%, but the actual rate is probably around 70%, similar to that recorded in previous outbreaks.9,10
The information available to us on the impact of the virus on the paediatric population is limited, since previous outbreaks have arisen in regions where resources are scarce and data on paediatric cases have not been routinely collected.
Since the current outbreak began we have been learning about the disease daily and the evidence is that children have the same risk of developing EVD as adults. In one of the few publications containing paediatric data, the fatality rate in children was high, and equal to that of adults, but when the results were stratified by age groups it was observed that children of school age showed a high survival rate when compared with children under five or with adults.11
In general fewer children have been identified with Ebola than adults. This is probably due to the fact that children are separated from sick people and are not involved in their care. Nor do they play an active part in preparing funeral rituals.
The signs and symptoms of EVD in children are similar to those in adults, but although information is limited, non-specific initial symptoms different from those in adults have been described in children. Fever tends to be constant (87–100% of cases). Initially it is associated with asthaenia, loss of appetite and cough, with nausea, vomiting and diarrhoea in the first week described in nearly 70% of cases, and less frequently irritability, headache, abdominal pain and sore throat. Fever therefore seems to be more common in the clinical presentation, as are gastrointestinal and respiratory symptoms, all of which are more common in children. On the other hand, haemorrhagic complications are less frequent (around 16%) in paediatric patients.12
Certain characteristics of the child population could worsen the condition of patients when they present with some of the initial symptoms of the infection. The younger children are, the more prone they are to become dehydrated by vomiting or diarrhoea. Their reserve of body fluids is smaller than that of adults, and this increases the risk of rapid dehydration, which is common with this disease. Children also have a lower volume of circulating blood that adults, and therefore without prompt intervention relatively small losses of blood can become more serious and affect haemodynamic balance more rapidly.
The pathogenesis of EVD in children could be different from that of adults, as a greater inflammatory reaction has been shown, and also the endothelial production of a substance (RANTES expression) that does not occur in adults and is associated with a higher survival rate. This could be a therapeutic target for the future.13 The problem is that these markers are very difficult to modify by therapeutic intervention.
The situation of pregnant women with EVD is one of extremely high fatality, both for the mother and for the newborn. Maternal mortality rates are nearly 95%, commonly with miscarriages in the first or second trimester and premature stillbirths in the last trimester (neonatal mortality close to 100%). It must be borne in mind that these data come from countries with scarce resources and that other concomitant diseases such as malaria, STD, HIV, etc. may possibly contribute to the high mortality.
There are other associated factors that distinguish cases of EVD in the paediatric population. Small children depend on their parents or other adults to meet their physical and psychological needs, and this poses a unique situation in terms of isolation and quarantine protocols. For example, if a case of a child with suspected EVD arose in Spain, only one of the parents could stay with the patient, provided that parent was informed and accepted the risk, and adopted individual protective measures. But if a suspected case were confirmed, what would we do with the parents? It is difficult to train parents quickly and ensure that they comply with all the rules on protection and proper use of personal protective equipment (PPE). Besides, they could not wear PPE all day, given the recommendation to limit its use to about 45–60min. All this means that the child would have to remain alone, in an isolation unit, which would oblige us to use sedation in order to be able to keep the child confined and reduce his or her stress. This point determines the care procedures that the paediatrician and the nurse will use. These decisions may vary, depending partly on the hospital protocols and the time of transmission of the disease, since the parents and carers may also be infected or have been previously exposed.
In the region affected by Ebola virus the impact of the crisis is much greater than is reflected by the data, not only because we know that there are undeclared cases of the disease, but also because urgent problems have been created relating to child protection, health infrastructure, education and means of subsistence, in a region where 22.3 million people live.
The paediatric population is always the most vulnerable in emergencies, and since the initial outbreak of EVD, children and their families have been exposed to situations of anxiety due to bereavement and family separation and subjected to isolation and to the general disorganisation of society. Being confined to the home through quarantine and witnessing the suffering of family members are especially painful experiences for children. Despite the efforts made to spread information in the affected areas, Ebola orphans and surviving children continue to face abandonment and stigmatisation in their communities. The quarantining of whole towns and villages makes it difficult to obtain adequate food, leading to an alarming increase in malnutrition. In addition, there are an increasing number of reports of EVD survivors who cannot even buy food at local markets, because people fear they may still be able to infect them.
The repercussions on the health of the child population take the form of an increase in mortality from malaria, diarrhoea and respiratory and other infections,14 due to reduced access to Health Centres, many of which have been closed since the start of the epidemic, and reluctance to attend health care facilities for fear of being infected. There is no access to education, because the schools were closed by government order as a preventive measure as soon as the crisis was declared. UNICEF estimates that the closure of schools has affected the education of over 4 million children.15 We know from other crises that once children abandon school, many of them never return and they run a high risk of becoming involved in child labour and other kinds of exploitation.
In Spain, the decision to repatriate two missionaries, both health care workers, in a serious clinical condition and in an advanced stage of EVD, who died a few days after being transferred and admitted to the Carlos III Hospital in Madrid, was difficult and questionable. Decisions were obviously taken under intense political and media pressure and quite probably with a certain degree of improvisation, especially as regards where the two patients were placed (the Carlos III Hospital), bearing in mind its state of preparedness and the availability, at the time, of staff properly trained to look after patients suffering from a highly transmissible disease with a high mortality rate. Probably nobody in Spain was adequately prepared to care for these patients at that time. The infection of the nursing assistant in Madrid from the second repatriated case precipitated a serious crisis situation and increased the level of uncertainty and distrust over the safety of the protocols. We “hit rock bottom”, so to speak. Something similar occurred in the United States, arising from the secondary cases in Dallas, which obliged the CDC to revise and completely rethink its strategy and prevention measures.
In the Spanish hospitals designated to treat cases of EVD we have been working and continue to work intensively to update and adapt our protocols and to improve the level of training of the health care staff responsible for the care of cases of EVD under investigation and the level of information of the rest of the hospital staff, so that we work safely and carry on as normal when we admit a patient with suspected EVD. It has not been and is not easy. To paraphrase an American colleague, “we’re building the boat while sailing”.
Moreover, the possibility of treating paediatric cases or pregnant patients with suspected EVD in our hospitals poses a series of additional problems which make it even more complex, if that is possible. In our experience, the best way of working is to establish mixed, versatile teams that include paediatric doctors and nurses together with doctors and nurses specialising in intensive care. In addition to these kinds of clinical care staff, it is vitally important that teams pay close attention to incorporating ancillary health care staff and cleaning, maintenance and security staff, for example. The environment (the care unit) must be safe and must have sufficient space and resources to carry out any task, however simple it may seem, under direct supervision (observation) and indirect supervision (recording with video cameras). Attention has already been drawn to the extreme importance of training in putting on and removing the current Personal Protection Equipment, which is certainly uncomfortable and which does not allow the care process to be performed completely as normal or for many minutes at a time. Every entry into the isolation unit must be carefully scheduled, planned and conducted according to protocol. Specific training in the various clinical situations that are most likely to occur (from simple patient hygiene to intubation and mechanical ventilation) must be intensive and must be conducted under real working conditions. The safety of the professionals (the main objective of any care process in these circumstances) depends on the resources available, on the training received and especially on confidence in good teamwork, with adequate supervision of each and every one of the actions performed by each and every member of the team.
In the Mixed Unit formed by the Hospital de Sant Joan de Deu and the Hospital Clínic in Barcelona, certified by the Ministry of Health (MSSSI) as a Referral Centre (CSUR) for Imported Tropical Diseases, we have adopted a model in which all the professionals belonging to the care team for each case of EVD under investigation (and of any other transmissible disease with a high biological hazard level) are volunteers, properly trained and prepared. This team essentially includes physicians and nurses specialising in Paediatrics, Intensive Care, Infectious Diseases and Tropical Diseases. There are a further two supplementary teams: a specialised team (of Obstetricians and Neonatologists) capable of offering care to pregnant patients with suspected EVD (including possible delivery) and a team that ensures care can be provided for cases of suspected EVD requiring surgical assessment or involving an orthopaedic problem. Both teams include specialists in Anaesthesiology and Resuscitation. These two situations (patients with suspected EVD who are pregnant or in labour and surgical patients with suspected EVD) are unlikely to occur in our setting, but it was considered necessary to establish and train these teams (also made up of volunteers) to cover that eventuality, which has meant formulating and applying specific protocols (no easy task in the case of a pregnant patient given the additional risk involved in exposure to blood and body fluids during delivery) and carrying out specific simulations.
In West Africa the epidemic seems to have entered a phase of better control (with a sustained decrease in the number of cases), though to varying degrees. Liberia has achieved a clear reduction in the number of new cases, Guinea-Conakry shows much more irregular progress, with periodic rises and falls in the number of cases, and Sierra Leone has the worst figures, with an increase, albeit more moderate, perhaps, in the number of cases. Nigeria, Senegal and Mali have brought the localised outbreaks originating from imported cases under control and have been declared free of the disease. The United States and Spain have also been declared free of the disease for the same reason.
The current increase in aid, from official sources and various charity organisations, in the area of West Africa means increased likelihood of infection among expatriate workers or high-risk exposure, leading to medical evacuation of such workers, on the one hand, and an increased risk of detecting an imported case among these workers once they have returned to their country of origin after their period of service in the epidemic zone. Examples of the foregoing are what has already happened in Spain (the case of risk exposure in an MSF doctor) and in the United Kingdom (detection of a confirmed case in a nurse after her return from the epidemic area), as well as several other cases of repatriation of confirmed cases or high-risk exposures in several EU countries and in the USA.
For all these reasons we must not lower our guard. The virus will not be eradicated in West Africa this year (2015); international aid will continue to be insufficient and has not achieved the anticipated objectives. The negative economic consequences for these countries will be substantial; many children have lost months of schooling and the health care systems are in a state of collapse. The “collateral damage” will be very severe: more orphans and an increase in mortality from other causes, especially mother/infant mortality, for lack of health care.
We, from our undoubtedly privileged position, must try to fulfil two objectives: the first is to contribute significantly to controlling the epidemic at its source and helping to re-establish and strengthen the health care systems of the affected countries. The second is to ensure the safety and effectiveness of any rapid detection process here in Spain and health care for suspected cases of EVD (health workers, aid workers, travellers, immigrants, etc.) from the epidemic area. As long as there is an epidemic in Africa we will all be at risk.
Please cite this article as: Fumadó V, Trilla A. Enfermedad por virus Ébola: un año después. An Pediatr (Barc). 2015;82:125–128.