The aim of this study was to evaluate the Amiel-Tison neurological examination (AT) and cranial ultrasound at term for predicting the neurological result at 12 months in newborns with neurological risk.
Patients and methodsThe study included 89 newborns with high risk of neurological damage, who were discharged from the Neonatal Intensive Care of the Hospital Zonal Bariloche, Argentina.
The assessment consisted of a neurological examination and cranial ultrasound at term, and neurological examination and evaluation of development at 12 months. The sensitivity, specificity, positive and negative predictor value was calculated. The relationship between perinatal factors and neurodevelopment at 12 months of age was also calculated using logistic regression models.
ResultsSeventy children completed the follow-up. At 12 months of age, 14% had an abnormal neurological examination, and 17% abnormal development. The neurological examination and the cranial ultrasound at term had low sensitivity to predict abnormal neurodevelopment. At 12 months, 93% of newborns with normal AT showed normal neurological results, and 86% normal development. Among newborns with normal cranial ultrasound the percentages were 90 and 81%, respectively.
Among children with three or more perinatal risk factors, the frequency of abnormalities in the neurological response was 5.4 times higher than among those with fewer risk factors, and abnormal development was 3.5 times more frequent.
ConclusionsThe neurological examination and cranial ultrasound at term had low sensitivity but high negative predictive value for the neurodevelopment at 12 months. Three or more perinatal risk factors were associated with neurodevelopment abnormalities at 12 months of age.
El objetivo de este estudio fue valorar el examen neurológico de Amiel-Tison (AT) y la ecografía cerebral a término para predecir el resultado neurológico a los 12 meses en recién nacidos con riesgo neurológico.
Población y métodosIngresaron en el estudio 89 niños con riesgo neurológico internados en Cuidados Intensivos Neonatales del Hospital Zonal Bariloche. La evaluación comprendió AT y ecografía cerebral neonatal y a los 12 meses, AT y valoración del desarrollo.
Se calculó sensibilidad, especificidad, valour predictivo positivo y negativo de las evaluaciones neonatales en relación al neurodesarrollo a los 12 meses. Se usó el modelo de regresión logística para evaluar la asociación entre factores perinatales y neurodesarrollo.
ResultadosCompletaron el estudio 70 niños, de los cuales un 14% presentó examen neurológico anormal y un 17% desarrollo anormal a los 12 meses. AT y ecografía cerebral neonatal tuvieron baja sensibilidad para estimar neurodesarrollo a los 12 meses. Entre neonatos con AT normal un 93% presentó resultado neurológico normal y un 86% desarrollo normal a los 12 meses; en aquellos con ecografía normal los porcentajes fueron 90 y 81%, respectivamente. Los neonatos con tres o más factores de riesgo presentaron 5,4 veces más posibilidad de AT anormal a los 12 meses que aquellos con menos factores y 3,5 veces más posibilidad de prueba de desarrollo anormal.
ConclusionesEl examen neurológico y la ecografía cerebral neonatales tienen baja sensibilidad pero alto valour predictivo negativo para neurodesarrollo a los 12 meses. Tres o más factores de riesgo se asocian a neurodesarrollo anormal.
Advances in perinatal care have increased the survival of high-risk newborns such as those with very low birth weights or suffering from neonatal encephalopathy. Prenatal care, the widespread use of prenatal steroids and surfactant therapy and the standardisation of resuscitation and intensive care techniques have resulted in a significant reduction of neonatal mortality worldwide.1,2 In developing countries, the impact on survival has been less pronounced, mostly due to the lack of access of part of the population to adequate paediatric care. In Argentina, there are significant differences in the rates of survival and complications between neonatal intensive care units (NICUs) in different regions.3–5
The nervous system is very vulnerable in newborns. Ill newborns are subjected to aggressive treatments that may carry collateral damage. At-risk neonates may develop severe neurological sequelae, such as cerebral palsy, intellectual disability, epilepsy and neurosensory disorders, as well as less severe complications like impaired motor coordination, attention deficit hyperactivity disorder and impairments in learning, language, attention, and spatial processing, among others.6 Severe sequelae manifest in early childhood, while those that are less severe appear later in life.7–10 These sequelae may have a negative impact on the academic performance and the cognitive and social skills of the child.
One of the chief concerns of the teams involved in the followup of these children is the early identification of those that may develop sequelae for the purposes of implementing early interventions to improve outcomes.8,10,11 Numerous studies have assessed different tools for predicting neurological outcomes. Brain lesions detected in the neonatal period by cranial ultrasound can predict various impairments of neuromotor and cognitive development.12–15 Neurological examinations such as the Amiel-Tisson and the Dubowitz assessments performed in the neonatal period are associated with neurological outcomes in at-risk newborns.16,17
Pregnancy or labour complications and neonatal morbidity are associated with abnormal neurological outcomes in the short and long term.18,19
The aim of our study was to analyse the predictive value of neurological assessment and cranial ultrasound for neuromotor and cognitive development outcomes at 12 months in a population of high-risk newborns admitted to the NICU of the Hospital Zonal Bariloche. We also analysed the association between perinatal and maternal factors and neurological outcome at 12 months.
Population and methodsWe conducted an observational prospective study. The study included every child born between August 2009 and June 2012 admitted to the NICU of the Hospital Zonal Bariloche with neurological risk.
The risk of neurological damage was defined as a gestational age of 32 weeks or lower, a birth weight of 1500g or less, and the presence of abnormal signs in the neurological assessment performed within a few days from birth, irrespective of gestational age.
We excluded newborns with chromosomal abnormalities and congenital malformations.
The Hospital Zonal Bariloche is a general hospital serving the Andean region of the province of Río Negro in the Argentine Patagonia.
The study was approved by the ethics committee of the Hospital Zonal Bariloche. We invited the parents to participate in the study in the early days of their child's stay, providing them with information about the project and requesting their consent.
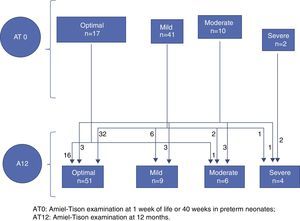
We evaluated the infants from the time they were admitted to the NICU to 12 months of corrected age. The evaluation consisted of neurological assessments, cranial ultrasounds and developmental assessments. Neurological assessments were carried out by Fabiana Herbón using the Amiel-Tison20,21 method at 1 week post-birth in term neonates or at 40 weeks of gestational age in premature neonates (AT0), and at 6 and 12 months of age (AT12). The neurological response was classified as optimal or with abnormal findings, the latter being further classified into mild, moderate or severe according to the criterion established for each age.22
The first cranial ultrasound was performed within 72h of admission, and followup was performed based on clinical judgement. In our analysis, we used the first ultrasound performed in term neonates and at 40 weeks of gestational age in preterm neonates. The images were classified into normal or abnormal by Jorge Moguilevsky. They were considered abnormal if they showed at least one of the following: intraventricular haemorrhage, periventricular hyperechogenicity (nonspecific signs of oedema, cystic periventricular leukomalacia and echolucencies).
The developmental assessment was performed at 12 months by FH by means of the Clinical Adaptive Test Clinical Linguistic and Auditory Milestone Scale (CAT–CLAMS)23,24 which evaluates problem solving skills (CAT) and language development (CLAMS). These assessments are summarised into a score, based on which, development is categorised as normal, at risk and delayed.23,24
The perinatal risk factors under consideration were birth weight below 1000g, requiring mechanical ventilation, bronchopulmonary dysplasia, sepsis, seizures and the lack of prenatal steroid treatment in infants born preterm at less than 35 weeks of gestation. The maternal factors included adolescent mother (age ≤19 years), an educational attainment at primary school level, and the presence of preeclampsia or of chorioamnionitis.
We studied the relationship between the observations in the neonatal period (AT0, cranial ultrasound, maternal history and perinatal risk factors) and the results of the Amiel-Tison assessment and the CAT–CLAMS at 12 months. For the purposes of the analysis, we grouped AT12 results into two categories: normal (optimal and mild abnormality) and abnormal (moderate or severe abnormality). A mild abnormality involves a subtle deviation from normality, moderate and severe abnormalities involve some degree of impairment at 12 months.22
The cranial ultrasound results were classified into normal and abnormal. The CAT–CLAMS results were classified as normal or abnormal (score <85).
We assessed the relationship of perinatal and maternal factors with the outcome at 12 months by means of logistic regression models with the following response variables: abnormal AT12 and abnormal CAT–CLAMS. We report the odds ratios and their 95% confidence intervals (CIs).
We analysed the association of the Amiel-Tison assessment and the ultrasound at term with the results at 12 months, calculating the sensitivity, specificity, positive predictive value and negative predictive value. We performed the statistical analysis with the R 3.0.1 software.25
ResultsEighty-nine newborns were included in the study. Ten of them died before age 12 months, and nine were lost to followup. Seventy infants completed the study, of which 78.6% had been born preterm and 21.4% at term.
The median gestational age of the deceased patients was 26 weeks (range, 24–35 weeks) and their median birth weight was 885g (range, 480–2500g). Seventy percent of the patients that died had weighed less than 1000g. At least one ultrasound was performed in each of the deceased patients, and 80% of these scans were abnormal.
We did not find differences in maternal educational attainment, being born to an adolescent mother, sex, bore in the hospital, and use of prenatal steroids between the deceased infants and the infants that completed followup.
Nine infants were lost to followup (10%). There were no significant differences in maternal or newborn characteristics, the incidence of abnormal results in the neurological assessment at term age, and the incidence of abnormal ultrasound results between the children that dropped out of the study and those that completed followup (Table 1). The median gestational age of the children lost to followup was 34 weeks (range, 26–41 weeks), while that of the children that completed followup was 32 weeks (range, 26–43 weeks); this difference was not statistically significant (P=.530 [Wilcoxon]).
Characteristics of the newborns that completed followup and those that dropped out of the study.
| Characteristic | Followup | P | |
|---|---|---|---|
| Completed(N=70) | Dropped out(N=9) | ||
| n (%) | n (%) | ||
| Maternal educational attainment: primary school | 39 (55.7) | 6 (66.7) | .789 |
| Adolescent mother | 20 (28.6) | 2 (22.2) | .996 |
| Not born at Zonal Bariloche | 16 (22.9) | 1 (11.1) | .707 |
| Male sex | 37 (52.9) | 6 (66.7) | .669 |
| Birth weight | .502 | ||
| 640–1000g | 10 (14.3) | 2 (22.2) | |
| 1001–1500 | 22 (31.4) | 1 (11.1) | |
| 1501–2500 | 19 (27.1) | 2 (22.2) | |
| 2501–4300 | 19 (27.1) | 4 (44.4) | |
| No prenatal steroid treatment* | 19 (38.0) | 2 (40.0) | 1.000 |
| Moderate or severe Amiel-Tison at term | 12 (17.1) | 3 (33.3) | .475 |
| Ultrasound at term | 29 (41.4) | 3 (33.3) | .916 |
Percentage distribution of maternal and newborn characteristics in the group that completed the study and the group lost to followup, and P-value for the comparison.
When we considered perinatal factors individually, none showed an association with neuromotor outcome at 12 months. However, the presence of moderate or severe abnormalities in the neurological response was 5.4 times (95% CI, 1.3–22.1) more frequent among children with three or more perinatal risk factors than among those with fewer than three factors. We also observed abnormal developmental outcomes at 12 months 3.5 times (95% CI, 1.0–12.6%) more frequently in children that had three or more risk factors. We did not find a significant association between maternal history and outcomes at 12 months.
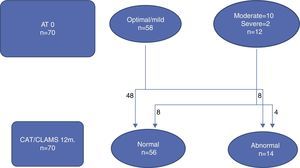
Figs. 1 and 2 illustrate the clinical course of the 70 children that completed followup. Of the 58 neonates with an optimal/mild AT0, 54 had an optimal/mild AT12 and 4 had an abnormal AT12, while of the 12 children that had a moderate/severe AT0, 6 went on to have an optimal/mild result and 4 remained in the moderate/severe group at 12 months (Fig. 1). We observed that when the developmental assessment was performed at 12 months, 8 of the 58 children with an optimal/mild AT0 had an abnormal development score, and that out of the 12 that had a moderate/severe AT0, 8 had a normal development score (Fig. 2).
Tables 2 and 3 show the sensitivity, specificity, and positive and negative predictive values of the two at-term assessments in relation to the results of the neurological and developmental assessments at 12 months. The sensitivity of AT0 in relation to the neurological response at 12 months is the percentage of children with a normal AT12 that had an abnormal AT0, while the specificity is the percentage of children with a normal AT12 that had a normal AT0. We also show the association between the neurological assessment results at 6 months and the AT12.
Predictability of neurological outcome at 12 months of corrected age based on the at-term neurological assessment and ultrasound.
| Method | Sn (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| AT0 | 60.0 (26.2–87.8) | 90.0 (79.5–96.2) | 50.0 (21.1–78.9) | 93.1 (83.3–98.1) |
| AT6 | 80.0 (44.4–97.5) | 91.7 (81.6–97.2) | 61.5 (31.6–86.1) | 96.5 (87.9–99.6) |
| Ultrasound | 60.0 (26.2–87.8) | 61.7 (48.2–73.9) | 20.7 (8.0–39.7) | 90.2 (76.9–97.3) |
| AT0 or ultrasound | 60.0 (26.2–87.8) | 95.0 (86.1–99.0) | 66.7 (29.9–92.5) | 93.4 (84.1–98.2) |
AT0, Amiel-Tison assessment at term; AT0 or ultrasound, normal if at least one of the two is normal; AT6, Amiel-Tison assessment at 6 months of corrected age; N=70; NPV, negative predictive value; PPV, positive predictive value; Sn, sensitivity; Sp, specificity.
Predictability of cognitive development at 12 months of corrected age based on the at-term neurological assessment and ultrasound.
| Method | Sn (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| AT0 | 33.3 (9.9–65.1) | 86.2 (74.6–93.9) | 33.3 (9.9–65.1) | 86.2 (74.6–93.9) |
| AT6 | 50.0 (21.1–78.9) | 87.9 (76.7–95.0) | 46.2 (19.2–74.9) | 89.5 (78.5–96.0) |
| Ultrasound | 33.3 (9.9–65.1) | 56.9 (43.2–69.8) | 13.8 (3.9–31.7) | 80.5 (65.1–91.2) |
| AT0 or ultrasound | 25.0 (5.5–57.2) | 89.7 (78.8–96.1) | 33.3 (7.5–70.1) | 85.2 (73.8–93.0) |
AT0, Amiel-Tison assessment at term; AT0 or ultrasound, normal if at least one of the two is normal; AT6, Amiel-Tison assessment at 6 months of corrected age; N=70; NPV, negative predictive value; PPV, positive predictive value; Sn, sensitivity; Sp, specificity.
The sensitivity of the AT0 and the cranial ultrasound are low for the results of both the neurological and the developmental assessments at 12 months. However, of all the children with a normal AT0, 93% (95% CI, 83–98%) had a normal neurological outcome and 86% (95% CI, 75–94%) a normal developmental outcome at 12 months. Of all children with a normal ultrasound at term, 90% (95% CI, 77–97%) had a normal neurological outcome and 81% (95% CI, 65–91%) showed normal development at 12 months. The positive predictive value of ultrasound was low (21%); however, of the 8 children with a high-risk result in the ultrasound at term, 75% had abnormal neurological outcomes at 12 months.
We observed an increase in specificity if at least one of the two results (ultrasound or neurological assessment) was normal (Tables 2 and 3).
DiscussionOur study assessed the predictability of neurodevelopment at 12 months of age based on cranial ultrasound and neurological assessment of at-risk neonates admitted to the NICU of the Hospital Zonal Bariloche.
As far as we know, there have been no studies investigating the prediction of neurological outcomes at 12 months in high-risk neonates in general hospitals in Argentina.
The neurological assessment at term showed a low sensitivity in predicting neurodevelopment at 12 months. However, of all newborns that had a normal neurological assessment, 93% had a normal neurological outcome and 86% a normal cognitive outcome at 12 months. Other authors have obtained similar results in different populations of at-risk neonates.16,17,26 The Amiel-Tison assessment at term was a better predictor of neurological outcome at 12 months than the developmental assessment.17 Paro-Panjan et al.27 found that normal results in the AT assessment in the neonatal period do not correlate strongly to developmental outcomes at 2 and 3 years of age.
Neonatal ultrasound had a low sensitivity for predicting both neurological and developmental outcomes at 1 year, corresponding to 60% and 33%, respectively. This is consistent with the findings of other studies.12,15,26 Of all children with a normal ultrasound, 90% had normal neurological outcomes and 81% had normal developmental outcomes at 12 months (negative predictive value). Kuban et al.15 assessed neonatal ultrasound as a tool for predicting cerebral palsy in preterm infants and found a sensitivity of 32% when ventriculomegaly was detected, and of 38% when there were echolucencies. The negative predictive value and the specificity were above 92%. O'Shea et al.12 studied the association of neonatal ultrasound and developmental outcome at 2 years in the same population. The presence of ventriculomegaly had a sensitivity of 17% both for psychomotor and cognitive development; while the negative predictive value ranged between 70% and 80% for both outcomes.
We used the CAT–CLAMS to assess development. While the Bayley Scales are usually employed to assess cognitive development in early childhood, due to the simplicity of its implementation the CAT–CLAMS is easier to apply in a regional hospital lacking staff accredited to perform the Bayley assessment. There is a strong correlation between the CAT–CLAMS and the Bayley II in the detection of developmental abnormalities at 12 months in high-risk or developmentally delayed children.28
We did not find an association between the individual occurrence of perinatal factors and neurodevelopment at 12 months. Some studies claim that the number or the combination of risk factors are the best outcome predictors.29–31 In our study, neonates with three or more perinatal risk factors were 5.4 times more likely to have an abnormal neurological response, and 3.5 times more likely to show an abnormal development than those with fewer risk factors.
There is evidence of the benefits of early intervention in outcomes at the short- and mid-term, especially in children of low socioeconomic status.32 Our population is predominantly of low socioeconomic status. Educational attainment was at the primary school level in 56% of the mothers, and 29% of mothers were adolescents.
The main limitation of this study is the reduced number of patients. The cohort consists of children with neurological risk, irrespective of gestational age, representative of the neonatal population in regional hospital NICUs.18 On the other hand, it proposes the use of two tools to assess the brain of high-risk neonates that have a high negative predictive value and can be implemented in any NICU with an intermediate level of care. Studies with a greater number of patients and with followups reaching through school age and adolescence are needed, as many neurodevelopmental problems do not manifest until later in life.6,7
The results of our study show that normal cranial ultrasound and Amiel-Tison neurological assessment results at term predict a normal neurological outcome at 12 months. However, if these results are abnormal, the prognosis is unclear, and other diagnostic tests such as magnetic resonance imaging have been shown to be ineffective.33 The number of perinatal risk factors should be taken into account for the purposes of prognosis, at least in the short term. Continuous long-term monitoring is irreplaceable in at-risk newborns in regional hospitals to implement early interventions and assess future neurological outcomes.
FundingThis study was partially funded by grant B188, Secretaría de Investigación, Universidad Nacional del Comahue.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Herbón F, Garibotti G, Moguilevsky J. Predicción temprana del resultado neurológico a los 12 meses en neonatos de riesgo en Bariloche. An Pediatr (Barc). 2015;83:123–129.