Unilateral renal agenesis, or solitary kidney, is a common disease (1/720 births) with a predominance of male patients. It affects the left kidney most frequently. Its aetiology and pathogenesis are unknown, with the literature describing possible genetic and environmental mechanisms.1 Given its frequent association with other malformations, some authors hypothesise that it may be part of a syndrome.1,2 The diagnosis is made by ultrasonography, usually before birth. Performance of a nuclear medicine assessment is recommended to rule out nephrourologic comorbidities. These patients are at higher risk of developing proteinuria, chronic kidney disease (CKD) and/or high blood pressure, and unilateral renal agenesis is a frequent cause of CKD in children aged less than 5 years.3,4 In this article, we describe our experience with this disease.
We conducted a retrospective, observational and descriptive study by collecting data from the health records of children born with unilateral renal agenesis in our hospital between 2008 and 2015. We found records for 21 patients (57% male) with a mean age of 3.8 years for the period under study. Forty-five percent of the patients had a prenatal diagnosis of unilateral renal agenesis, which was confirmed postnatally by renal ultrasound in all. Of the remaining 55% that received the diagnosis after birth, 66% had some type of malformation at birth (most frequently gastrointestinal), and underwent a renal ultrasound examination for the purpose of ruling out associated nephrourologic malformations (which were found in 45%, with a predominance of pyelocaliectasis [29%] and vesicoureteral reflux [VUR] [21%]). Other, less frequent reasons that led to diagnosis were oligohydramnios, spina bifida or acute pyelonephritis in the early days of life (11%).
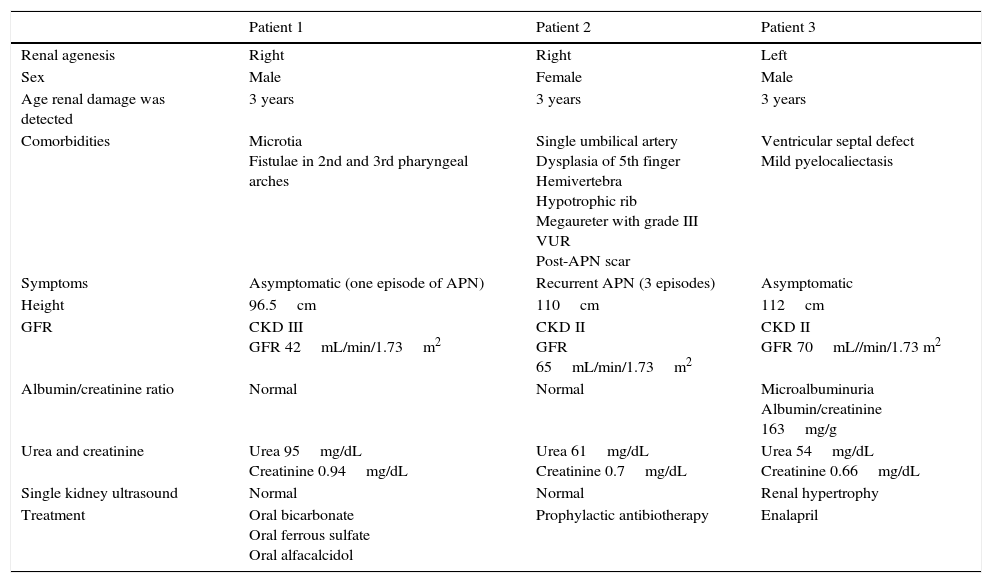
There were more cases of left-sided renal agenesis (65%), and a solitary hypertrophic kidney was detected in 55% of cases. All patients underwent a workup that included measurement of plasma urea and creatinine levels, urinary sediment analysis and calculation of the albumin/creatinine ratio, and the results were normal in all at the time of testing. The evaluation was completed with imaging of the kidney by 99mTc-mercaptoacetyltriglycine (MAG3) renography in 80% and serial voiding cystourethrography (VCUG) in 30% of patients. Eighty-one percent of patients were followed up in outpatient services (median duration, 3 years; range, 0–7 years), with periodic checkups including measurement of blood pressure, renal ultrasound examination, renal function panel, albumin/creatinine ratio, urinary sediment analysis and calculation of the glomerular filtration rate (GFR) using the Schwartz formula updated in 2009. We found no cases of high blood pressure. Thirty-five percent of patients had at least one episode of acute pyelonephritis that responded well to antibiotic treatment. Fifty percent of these patients had VUR and received prophylactic antibiotherapy. Three patients (17%) developed early CKD, associated with microalbuminuria in 1 (Table 1). None of the patients needed renal replacement therapy.
Data of patients with unilateral renal agenesis and development of kidney damage.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Renal agenesis | Right | Right | Left |
| Sex | Male | Female | Male |
| Age renal damage was detected | 3 years | 3 years | 3 years |
| Comorbidities | Microtia Fistulae in 2nd and 3rd pharyngeal arches | Single umbilical artery Dysplasia of 5th finger Hemivertebra Hypotrophic rib Megaureter with grade III VUR Post-APN scar | Ventricular septal defect Mild pyelocaliectasis |
| Symptoms | Asymptomatic (one episode of APN) | Recurrent APN (3 episodes) | Asymptomatic |
| Height | 96.5cm | 110cm | 112cm |
| GFR | CKD III GFR 42mL/min/1.73m2 | CKD II GFR 65mL/min/1.73m2 | CKD II GFR 70mL//min/1.73 m2 |
| Albumin/creatinine ratio | Normal | Normal | Microalbuminuria Albumin/creatinine 163mg/g |
| Urea and creatinine | Urea 95mg/dL Creatinine 0.94mg/dL | Urea 61mg/dL Creatinine 0.7mg/dL | Urea 54mg/dL Creatinine 0.66mg/dL |
| Single kidney ultrasound | Normal | Normal | Renal hypertrophy |
| Treatment | Oral bicarbonate Oral ferrous sulfate Oral alfacalcidol | Prophylactic antibiotherapy | Enalapril |
APN, acute pyelonephritis; CKD, chronic kidney disease; GFR, glomerular filtration rate; VUR, vesicoureteral reflux.
One of the salient findings of our study was the high morbidity associated with unilateral renal agenesis, which was consistent with the literature. For instance, Westland et al.5 analysed 2684 cases of unilateral renal agenesis in children and adults and found high blood pressure, microalbuminuria and chronic kidney disease with a GFR of less than 60mL/min/1.73m2 in 16%, 21% and 10% of participants, respectively (the estimated mean age of the onset of complications in the paediatric subset was 9.1 years). The development of these complications can be explained by the hyperfiltration hypothesis proposed by Brenner et al.6 on the basis of animal studies, according to which hypertrophy would be a compensatory adaptation to the reduced number of nephrons in individuals with unilateral renal agenesis.3,4 In our series, 17% of patients developed complications early (mean age, 3 years). Thirty-three percent of them also presented with a solitary hypertrophic kidney, while 66% had some type of associated nephrourologic abnormality (grade III VUR, pyelocaliectasis and/or recurrent acute pyelonephritis), which led us to believe that the early development of renal damage in our series may have been related not only to renal hypertrophy but also to the presence of these comorbidities. Furthermore, due to ethical concerns few studies on the hyperfiltration theory have been conducted in children, and all are case series with small sample sizes and highly variable and inconclusive results.4 Therefore, we believe that it is important to continue reporting cases and to perform longitudinal studies with larger samples in children with congenital unilateral renal agenesis with the purpose of elucidating the pathophysiology of this disease. Last of all, we ought to underscore that these patients may be asymptomatic, and they must be under followup with regular checkups for the early detection and treatment of complications, even in the absence of associated kidney or urologic malformations or acute pyelonephritis, as renal damage, as we found in our series, may be silent and occur in seemingly healthy patients.
Limitations of the studyDue to lack of documentation in the electronic health records, we could not obtain data on other factors that may be associated with less favourable outcomes in these patients, such as low birth weight, maternal use of pharmaceuticals during pregnancy or use of nephrotoxic drugs in the neonatal period, among others. Similarly, we do not know the reason for the performance of the abdominal ultrasound that led to postnatal diagnosis in 33% of patients.
Please cite this article as: Castellano-Martinez A, Rodriguez-Gonzalez M, Roldan-Cano V. Daño renal precoz en pacientes nacidos con agenesia renal unilateral. An Pediatr (Barc). 2017;87:171–173.