Pediatric spondylodiscitis (PSD) is an uncommon condition, for which there are no specific international clinical guidelines. Factors related to complications have not been stablished. Our aim was to describe clinical and epidemiological characteristics of PSD, to analyze factors associated with complications and to evaluate adherence to the recommendations of the Spanish National Consensus Document (NCD) for the diagnostic and therapeutic approach to acute osteoarticular infections.
Material and methodsAmbispective, multicenter, national study of two PSD cohorts: historical (2008–2012) and prospective (2015–2020, after publication of NCD).
Patients with diagnosis of PSD were included. Demographic, clinical, microbiological and radiological data were recorded. Factors related to the development of complications were analized by logistic regression. Comparisons between both cohorts were performed.
ResultsNinety-eight PSD were included. In 84.7%, diagnosis was confirmed by magnetic resonance imaging. Microbiological isolation was obtained in 6.1%, with methicillin-sensitive S. aureus as the main etiologic agent. Complications occurred in 18.9%, the most frequent being soft tissue abscess. Of the 8.6% of patients with sequelae, persistent pain was the most common. Comparing cohorts, there was better adherence to NCD treatment recommendations in the prospective one (57.6% vs. 12.9%, p < 0.01), including a reduction in the length of intravenous antibiotic therapy (10 vs. 14 days, p < 0.01).
ConclusionsThe evolution of PSD in our series was favorable, with low frequency of complications and sequelae. The adherence to the recommendations of the NCD was high. Studies with larger sample size are needed to establish new recommendations to optimize the approach to these infections.
La espondilodiscitis pediátrica (EDP) es una afección poco común, para la cual no existen guías clínicas internacionales específicas. No se han establecido factores relacionados con las complicaciones. Nuestro objetivo fue describir las características clínicas y epidemiológicas de la EDP, analizar los factores asociados al desarrollo de complicaciones y evaluar el cumplimiento de las recomendaciones del Documento de Consenso Nacional (DCN) español para el abordaje diagnóstico y terapéutico de las infecciones osteoarticulares agudas.
Material y métodosEstudio ambispectivo, multicéntrico, nacional de dos cohortes de EDP: histórica (2008–2012) y prospectiva (2015–2020, después de la publicación de DCN).
Se incluyeron pacientes con diagnóstico de EDP. Se registraron datos demográficos, clínicos, microbiológicos y radiológicos. Los factores relacionados con el desarrollo de complicaciones se analizaron mediante regresión logística. Se realizaron comparaciones entre ambas cohortes.
ResultadosSe incluyeron noventa y ocho EDP. En el 84,7% el diagnóstico se confirmó mediante resonancia magnética. Se obtuvo aislamiento microbiológico en el 6,1%, siendo S. aureus sensible a meticilina el principal agente etiológico. Las complicaciones ocurrieron en el 18,9%, siendo la más frecuente el absceso de tejidos blandos. Del 8,6% de los pacientes con secuelas, el dolor persistente fue el más frecuente. Al comparar las cohortes, hubo una mejor adherencia a las recomendaciones de tratamiento del DCN en la cohorte prospectiva (57,6% frente a 12,9%, p < 0,01), incluida una reducción en la duración de la terapia con antibióticos intravenosos (10 frente a 14 días, p < 0,01).
ConclusionesLa evolución de la EDP en nuestra serie fue favorable, con baja frecuencia de complicaciones y secuelas. La adherencia a las recomendaciones del DCN fue alta. Se necesitan estudios con mayor tamaño muestral que establezcan nuevas recomendaciones para optimizar el abordaje de estas infecciones.
Spondylodiscitis (SD) is an umbrella term encompassing those infections that involve the vertebrae and intervertebral discs, although it is frequently used to refer to all spinal infections, whether they are vertebral osteomyelitis (OM), isolated discitis or true SD.1–3 In addition to being infrequent diseases in childhood, their nonspecific manifestations, especially in younger children, poses a major diagnostic challenge for paediatricians,4 so that a high index of suspicion is required to avoid potential complications and sequelae arising from delayed diagnosis and treatment.2,5
The absence of abnormalities in conventional radiography until several weeks after the onset of the disease makes magnetic resonance imaging (MRI) the imaging test of choice for diagnosis of SD.3,6,7 In general, the isolation of a microorganism involved in the development of these infections would allow the most appropriate antibiotic treatment in each case; however, absence of microbiological isolation is the most common scenario in paediatric SD (PSD), occurring in up to 87%–100% of cases.2,8 This, together with the absence of standardized recommendations for the treatment of these infections in children,9 explains the considerable heterogeneity in the management of PSD in our field.
In 2014, the Spanish National Consensus Document (NCD) was published to address the diagnosis and treatment of uncomplicated acute osteoarticular infections (AOIs) in the paediatric age group,10,11 including few specific recommendations for PSD. Subsequent initiatives in countries in our region, including the guidelines of the European Society for Paediatric Infectious Diseases, have also attempted to establish recommendations for the treatment of these diseases in children.6,7,12
The Paediatrics Osteoarticular Infections Network (RIOPed, Red de Infecciones Ostearticulares Pediátricas), which currently includes 66 participating hospitals throughout Spain, has made it possible to collect data on paediatric AOI, retrospectively between 2008 and 2012 and prospectively since 2015, and to assess the impact of the NCD on the management of septic arthritis and acute osteomyelitis (AOM) in Spain one year after its publication.13
Material and methodsThe primary objective of the study was to describe the clinical and epidemiological characteristics of PSD and to analyse the possible factors associated with the development of complications in these patients. The secondary objectives were to evaluate adherence to the diagnostic and treatment recommendations included in the NCD for PSD and to assess the need for new recommendations that could optimize the management of these infections.
We conducted an ambidirectional, multicentre, nationwide study in the framework of the RIOPed in 2 two cohorts of patients with PSD aged less than 16 years, with participation of 22 Spanish hospitals in the historical cohort (2008–2012) and 37 in the prospective cohort (September 2015–May 2020, after the publication of the NCD).
The study was approved by the Ethics Committee of the Hospital Universitario Severo Ochoa and ratified by the participating centres.
The sample included patients with a diagnosis of PSD, defined as the presence of suggestive clinical features (fever, back pain, limping, refusal to sit or walk), with a compatible bone scan and/or MRI performed, and adequate response to antimicrobial treatment.
Complications were defined as the development of pyomyositis, soft tissue abscess, intramedullary abscess, neurological deficits, deep vein thrombosis, septic emboli or pneumonia.
We conducted a descriptive study of the two cohorts and analysed the factors associated with the development of complications in both cohorts combined.
We assessed for differences before and after the publication of the NCD and the degree of adherence to the diagnostic and treatment recommendations proposed in the NCD. We calculated the percentage of adherence to the recommendations that the expert panel considered mandatory: collection of sample for blood culture (BC), plain X-ray and MRI. We also assessed the choice of antibiotic treatment (empirical and targeted) and its duration (Table 1).
Recommendations for treatment of osteoarticular infections established in the National Consensus Document.
| Intravenous treatment | <3 months: cloxacillin + cefotaxime/gentamicin |
| 3 months-5 years: cefuroxime or cloxacillin + cefotaxime | |
| >5 years: cefazolin or cloxacillin | |
| Oral treatment | Newborn-3 months: cefuroxime axetil. Alternative: amoxicillin-clavulanic acid |
| 3 months-5 years: cefuroxime axetil or cefadroxil (especially in >2 years). | |
| > 5 years: cefadroxil | |
| Specific treatment | MSSA: cefadroxil |
| MRSA: clindamycin/ciprofloxacin/TMP-SMX +/− rifampicin | |
| S. pyogenes, S. agalactiae and S. pneumoniae: amoxicillina | |
| Kingella kingae: amoxicillin as first choice. Cefuroxime or amoxicillin clavulanate |
MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; TMP-SMX, trimethoprim-sulfamethoxazole.
Qualitative variables were summarised as frequencies and percentages and quantitative variables as median and interquartile range (IQR) after verifying that they did not follow a normal distribution by means of the Kolmogorov-Smirnov test.
We assessed for differences in the percentage distribution of qualitative variables using the χ2 test or the Fisher exact test, as appropriate. The comparison between column proportions for more than two categories was adjusted with the Bonferroni correction.
We compared continuous variables between groups with the Mann–Whitney U and Kruskal–Wallis tests depending on the number of compared categories.
To evaluate factors related to complications, we conducted a multivariate analysis using logistic regression, including those variables with a statistically significant association in the bivariate analysis or considered clinically relevant by the researchers.
The statistical analysis was carried out with the statistical package SPSS version 25.0. We considered p values of less than 0.05 (two-tailed) statistically significant.
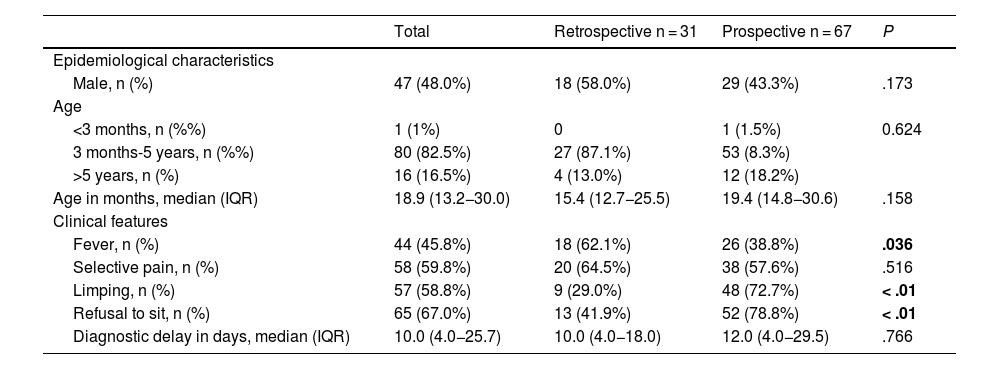
ResultsOf the 1701 patients with AOIs, 98 (5.8%) received a diagnosis of PSD, of whom 31 (31.6%) belonged to the retrospective cohort and 67 (68.4%) to the prospective cohort. The location of the PSD was lumbar in 52 cases (53.1%), dorsal in 8 (8.2%) and cervical in 7 (7.1%), and was not documented in 31 patients (31.6%). We found a higher frequency of fever in the retrospective cohort (62.1% vs. 38.8%; P = .036) and of limping and refusal to sit in the prospective cohort (29.0% vs. 72.7% and 41.9% vs. 78.8%, respectively; P = .01), with no differences between the two cohorts in any of the remaining epidemiological and clinical characteristics (Table 2). Table 3 shows the performed diagnostic tests, treatment and outcomes for both cohorts.
Comparison of the clinical and epidemiological characteristics of spondylodiscitis in the retrospective and prospective cohorts.
| Total | Retrospective n = 31 | Prospective n = 67 | P | |
|---|---|---|---|---|
| Epidemiological characteristics | ||||
| Male, n (%) | 47 (48.0%) | 18 (58.0%) | 29 (43.3%) | .173 |
| Age | ||||
| <3 months, n (%%) | 1 (1%) | 0 | 1 (1.5%) | 0.624 |
| 3 months-5 years, n (%%) | 80 (82.5%) | 27 (87.1%) | 53 (8.3%) | |
| >5 years, n (%) | 16 (16.5%) | 4 (13.0%) | 12 (18.2%) | |
| Age in months, median (IQR) | 18.9 (13.2−30.0) | 15.4 (12.7−25.5) | 19.4 (14.8−30.6) | .158 |
| Clinical features | ||||
| Fever, n (%) | 44 (45.8%) | 18 (62.1%) | 26 (38.8%) | .036 |
| Selective pain, n (%) | 58 (59.8%) | 20 (64.5%) | 38 (57.6%) | .516 |
| Limping, n (%) | 57 (58.8%) | 9 (29.0%) | 48 (72.7%) | < .01 |
| Refusal to sit, n (%) | 65 (67.0%) | 13 (41.9%) | 52 (78.8%) | < .01 |
| Diagnostic delay in days, median (IQR) | 10.0 (4.0−25.7) | 10.0 (4.0−18.0) | 12.0 (4.0−29.5) | .766 |
IQR, interquaratile range.
Variables with statistically significant differences are presented in boldface.
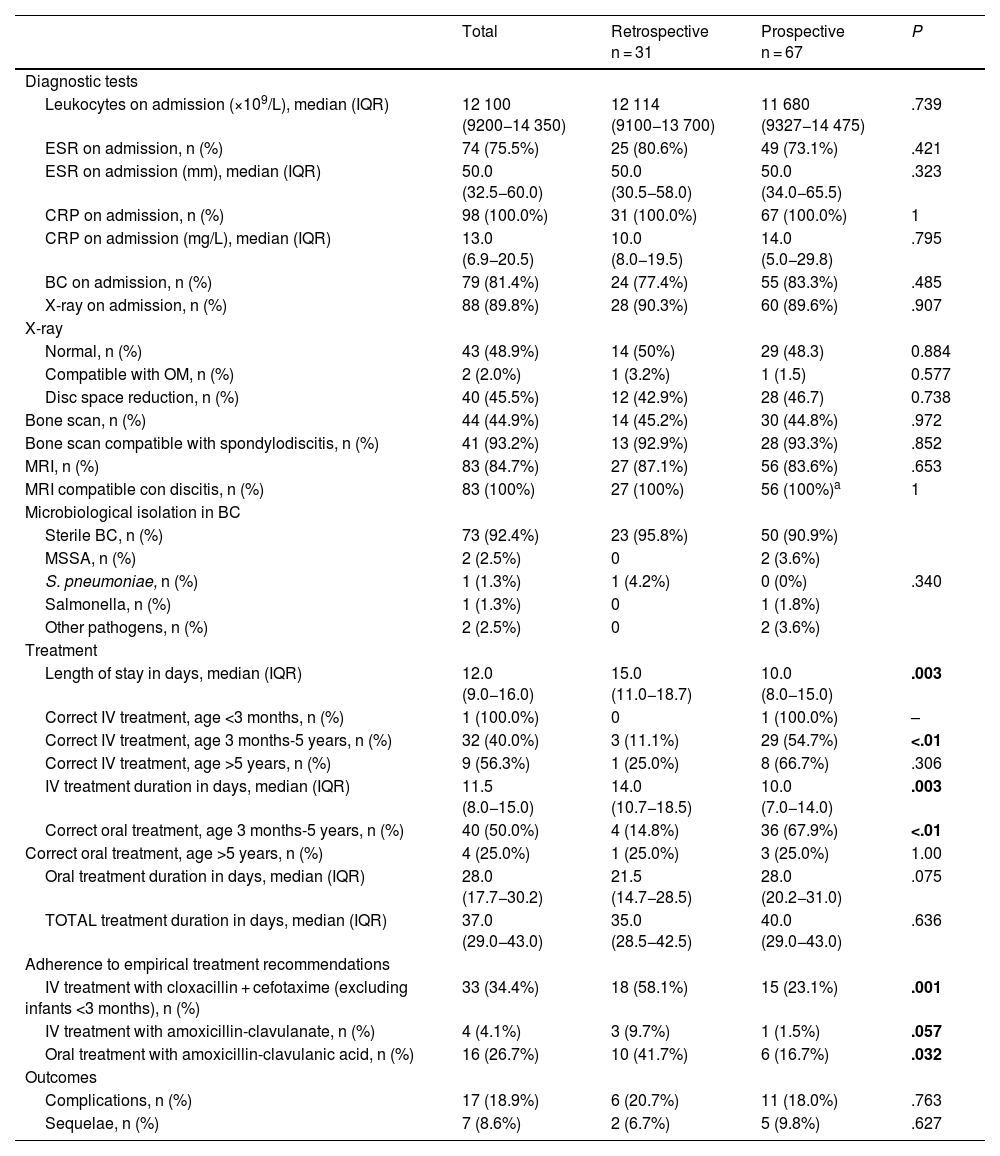
Comparison of diagnostic, therapeutic and evolution characteristics of spondylodiscitis between the retrospective and prospective cohorts.
| Total | Retrospective n = 31 | Prospective n = 67 | P | |
|---|---|---|---|---|
| Diagnostic tests | ||||
| Leukocytes on admission (×109/L), median (IQR) | 12 100 (9200−14 350) | 12 114 (9100−13 700) | 11 680 (9327−14 475) | .739 |
| ESR on admission, n (%) | 74 (75.5%) | 25 (80.6%) | 49 (73.1%) | .421 |
| ESR on admission (mm), median (IQR) | 50.0 (32.5−60.0) | 50.0 (30.5−58.0) | 50.0 (34.0−65.5) | .323 |
| CRP on admission, n (%) | 98 (100.0%) | 31 (100.0%) | 67 (100.0%) | 1 |
| CRP on admission (mg/L), median (IQR) | 13.0 (6.9−20.5) | 10.0 (8.0−19.5) | 14.0 (5.0−29.8) | .795 |
| BC on admission, n (%) | 79 (81.4%) | 24 (77.4%) | 55 (83.3%) | .485 |
| X-ray on admission, n (%) | 88 (89.8%) | 28 (90.3%) | 60 (89.6%) | .907 |
| X-ray | ||||
| Normal, n (%) | 43 (48.9%) | 14 (50%) | 29 (48.3) | 0.884 |
| Compatible with OM, n (%) | 2 (2.0%) | 1 (3.2%) | 1 (1.5) | 0.577 |
| Disc space reduction, n (%) | 40 (45.5%) | 12 (42.9%) | 28 (46.7) | 0.738 |
| Bone scan, n (%) | 44 (44.9%) | 14 (45.2%) | 30 (44.8%) | .972 |
| Bone scan compatible with spondylodiscitis, n (%) | 41 (93.2%) | 13 (92.9%) | 28 (93.3%) | .852 |
| MRI, n (%) | 83 (84.7%) | 27 (87.1%) | 56 (83.6%) | .653 |
| MRI compatible con discitis, n (%) | 83 (100%) | 27 (100%) | 56 (100%)a | 1 |
| Microbiological isolation in BC | ||||
| Sterile BC, n (%) | 73 (92.4%) | 23 (95.8%) | 50 (90.9%) | .340 |
| MSSA, n (%) | 2 (2.5%) | 0 | 2 (3.6%) | |
| S. pneumoniae, n (%) | 1 (1.3%) | 1 (4.2%) | 0 (0%) | |
| Salmonella, n (%) | 1 (1.3%) | 0 | 1 (1.8%) | |
| Other pathogens, n (%) | 2 (2.5%) | 0 | 2 (3.6%) | |
| Treatment | ||||
| Length of stay in days, median (IQR) | 12.0 (9.0−16.0) | 15.0 (11.0−18.7) | 10.0 (8.0−15.0) | .003 |
| Correct IV treatment, age <3 months, n (%) | 1 (100.0%) | 0 | 1 (100.0%) | – |
| Correct IV treatment, age 3 months-5 years, n (%) | 32 (40.0%) | 3 (11.1%) | 29 (54.7%) | <.01 |
| Correct IV treatment, age >5 years, n (%) | 9 (56.3%) | 1 (25.0%) | 8 (66.7%) | .306 |
| IV treatment duration in days, median (IQR) | 11.5 (8.0−15.0) | 14.0 (10.7−18.5) | 10.0 (7.0−14.0) | .003 |
| Correct oral treatment, age 3 months-5 years, n (%) | 40 (50.0%) | 4 (14.8%) | 36 (67.9%) | <.01 |
| Correct oral treatment, age >5 years, n (%) | 4 (25.0%) | 1 (25.0%) | 3 (25.0%) | 1.00 |
| Oral treatment duration in days, median (IQR) | 28.0 (17.7−30.2) | 21.5 (14.7−28.5) | 28.0 (20.2−31.0) | .075 |
| TOTAL treatment duration in days, median (IQR) | 37.0 (29.0−43.0) | 35.0 (28.5−42.5) | 40.0 (29.0−43.0) | .636 |
| Adherence to empirical treatment recommendations | ||||
| IV treatment with cloxacillin + cefotaxime (excluding infants <3 months), n (%) | 33 (34.4%) | 18 (58.1%) | 15 (23.1%) | .001 |
| IV treatment with amoxicillin-clavulanate, n (%) | 4 (4.1%) | 3 (9.7%) | 1 (1.5%) | .057 |
| Oral treatment with amoxicillin-clavulanic acid, n (%) | 16 (26.7%) | 10 (41.7%) | 6 (16.7%) | .032 |
| Outcomes | ||||
| Complications, n (%) | 17 (18.9%) | 6 (20.7%) | 11 (18.0%) | .763 |
| Sequelae, n (%) | 7 (8.6%) | 2 (6.7%) | 5 (9.8%) | .627 |
BC, blood culture; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range; MRI, magnetic resonance imaging; MSSA, methicillin-sensitive S. aureus; OM, osteomyelitis.
Variables with statistically significant differences are presented in boldface.
Overall, complications occurred in 18.9% of patients (n = 17), with soft tissue abscess being the most frequent one (58.8%) followed by pyomyositis (23.6%). Two patients developed intramedullary abscess (11.8%). There was no evidence of other complications. Sequelae were observed in 8.6% of the patients (n = 7), of which the most frequent was persistent residual pain (57.1%), followed by limping (14.3%). There were no differences between the two cohorts in the percentages of complications and sequelae (Table 3).
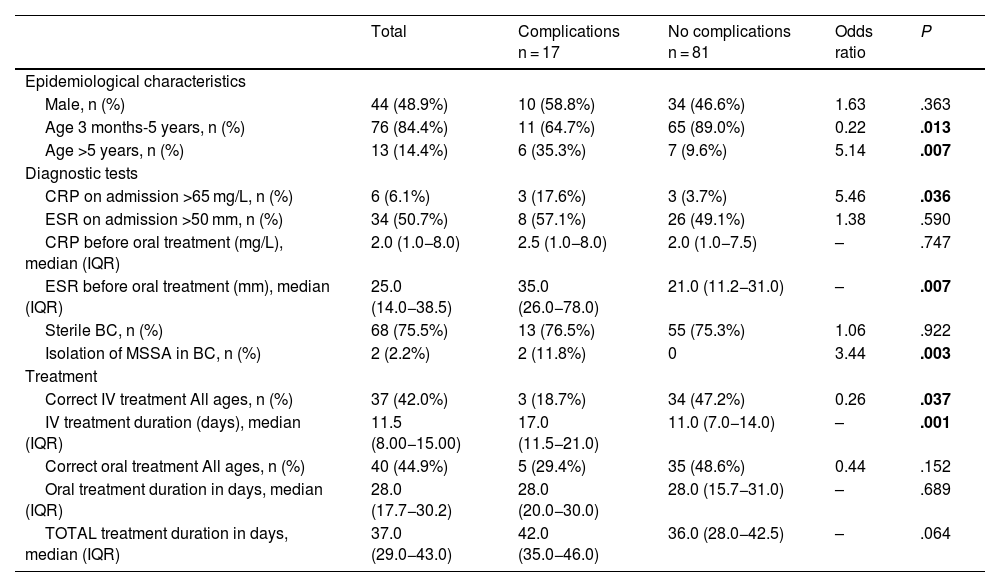
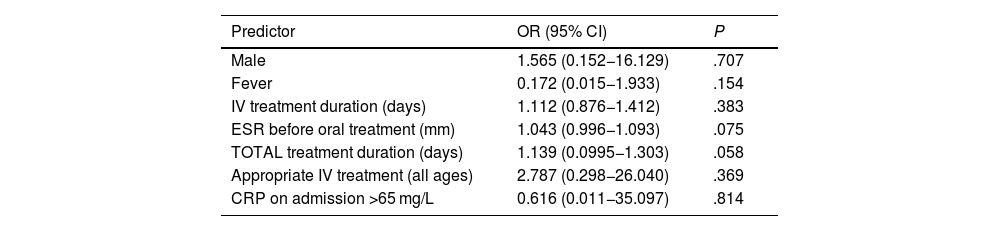
In the bivariate analysis, age greater than 5 years, a C-reactive protein (CRP) level greater than 65 mg/L on admission, a higher erythrocyte sedimentation rate (ESR) before oral treatment, isolation of methicillin-sensitive Staphylococcus aureus (MSSA) in blood culture or incorrect treatment (relative to the recommendations of the NCD) were associated with an increased risk of complications (Table 4). In the multivariate analysis, none of the analysed variables were associated with an increased risk of complications (Table 5).
Factors associated with the development of complications in paediatric spondylodiscitisa (bivariate analysis).
| Total | Complications n = 17 | No complications n = 81 | Odds ratio | P | |
|---|---|---|---|---|---|
| Epidemiological characteristics | |||||
| Male, n (%) | 44 (48.9%) | 10 (58.8%) | 34 (46.6%) | 1.63 | .363 |
| Age 3 months-5 years, n (%) | 76 (84.4%) | 11 (64.7%) | 65 (89.0%) | 0.22 | .013 |
| Age >5 years, n (%) | 13 (14.4%) | 6 (35.3%) | 7 (9.6%) | 5.14 | .007 |
| Diagnostic tests | |||||
| CRP on admission >65 mg/L, n (%) | 6 (6.1%) | 3 (17.6%) | 3 (3.7%) | 5.46 | .036 |
| ESR on admission >50 mm, n (%) | 34 (50.7%) | 8 (57.1%) | 26 (49.1%) | 1.38 | .590 |
| CRP before oral treatment (mg/L), median (IQR) | 2.0 (1.0−8.0) | 2.5 (1.0−8.0) | 2.0 (1.0−7.5) | – | .747 |
| ESR before oral treatment (mm), median (IQR) | 25.0 (14.0−38.5) | 35.0 (26.0−78.0) | 21.0 (11.2−31.0) | – | .007 |
| Sterile BC, n (%) | 68 (75.5%) | 13 (76.5%) | 55 (75.3%) | 1.06 | .922 |
| Isolation of MSSA in BC, n (%) | 2 (2.2%) | 2 (11.8%) | 0 | 3.44 | .003 |
| Treatment | |||||
| Correct IV treatment All ages, n (%) | 37 (42.0%) | 3 (18.7%) | 34 (47.2%) | 0.26 | .037 |
| IV treatment duration (days), median (IQR) | 11.5 (8.00−15.00) | 17.0 (11.5−21.0) | 11.0 (7.0−14.0) | – | .001 |
| Correct oral treatment All ages, n (%) | 40 (44.9%) | 5 (29.4%) | 35 (48.6%) | 0.44 | .152 |
| Oral treatment duration in days, median (IQR) | 28.0 (17.7−30.2) | 28.0 (20.0−30.0) | 28.0 (15.7−31.0) | – | .689 |
| TOTAL treatment duration in days, median (IQR) | 37.0 (29.0−43.0) | 42.0 (35.0−46.0) | 36.0 (28.0−42.5) | – | .064 |
BC, blood culture; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range.
Variables with statistically significant differences are presented in boldface.
Factors associated with the development of complications in paediatric spondylodiscitis (multivariate analysis).
| Predictor | OR (95% CI) | P |
|---|---|---|
| Male | 1.565 (0.152−16.129) | .707 |
| Fever | 0.172 (0.015−1.933) | .154 |
| IV treatment duration (days) | 1.112 (0.876−1.412) | .383 |
| ESR before oral treatment (mm) | 1.043 (0.996−1.093) | .075 |
| TOTAL treatment duration (days) | 1.139 (0.0995−1.303) | .058 |
| Appropriate IV treatment (all ages) | 2.787 (0.298−26.040) | .369 |
| CRP on admission >65 mg/L | 0.616 (0.011−35.097) | .814 |
CI, confidence interval; CPR, C-reactive protein C; ESR, erythrocyte sedimentation rate; OR, odds ratio.
As regards the diagnostic recommendations given in the NCD, there were no differences in the frequency of imaging tests between both cohorts (Table 3). There was no decrease in the use of bone scintigraphy as a diagnostic test, with a yield for PSD detection of 92.9% in the retrospective cohort and 93.3% in the prospective cohort. The main etiological agent was MSSA, identified in 2 patients aged 18 months and 13 years. In addition, Streptococcus pneumoniae was isolated in a patient aged 1 year and Salmonella spp in a patient aged 11 years, both without known risk factors. All the isolates were obtained from BC samples. Bone biopsy with culture was performed in 2 patients of the prospective cohort (3.1%), but microbiological identification was not achieved. It was not possible to compare the cost-effectiveness of this technique in relation to the retrospective phase due to the lack of data on this variable in the historical cohort.
The impact that the recommendations of the NCD had on intravenous treatment was evident in the prospective cohort, as it was correct in 57.6% of the patients compared to 12.9% in the retrospective cohort (P < .01), mainly in the subgroup of patients aged 3 months to 5 years (54.7% vs. 11.1%; P < .01), while the difference was not statistically significant in the subgroup of patients aged more than 5 years, although the same trend was observed (66.7% in the prospective cohort vs. 25.0% in the retrospective cohort; P = .306). The use of combined intravenous regimens (cefotaxime + cloxacillin) in patients aged more than 3 months was significantly reduced in the prospective cohort (23.1% vs. 58.1%; P < .01), and there was also a decrease in the use of intravenous amoxicillin-clavulanic acid after the publication of the NCD (1.5% vs. 9.7%; P = .057). The length of stay and the duration of intravenous treatment were shorter in the prospective cohort (10 vs. 15 days [P < .01] and 10 vs. 14 days [P < .01], respectively).
Similarly, the adherence to the NCD oral treatment recommendations was greater in the prospective cohort (60.0% vs. 16.1%; P < .01), especially in the subgroup of patients aged 3 months to 5 years (67.9 vs. 14.8%; P < .01). There were no differences in the adherence to oral treatment recommendations in patients older than 5 years (Table 3). The use of oral amoxicillin-clavulanic acid for empirical treatment decreased from 38.7% in the retrospective cohort to 13.4% in the prospective cohort (P < .01). The duration of oral antibiotic therapy was longer in the prospective cohort (28.0 vs. 21.5 days; P < .07), and there were no differences in the total duration of treatment between the two cohorts (Table 3).
DiscussionDue to the lack of guidelines providing recommendations for the diagnosis and treatment of PSD, clinicians need to extrapolate the recommendations given for other AOIs, which leads to great variability in the management of PSD. All this makes it difficult to analyse outcomes and implement new recommendations to optimize the diagnostic and therapeutic approach. The development of the NCD10,11 made it possible to standardise the general approach to AOM, irrespective of its location. This case series shows the impact that this document has had on clinical practice, allowing a more uniform management of PSD in the prospective cohort.
In our study, both cohorts had similar epidemiological and clinical characteristics. The differences in the presence of fever, limping and refusal to sit may be due to improved documentation in the prospective cohort or to the higher mean age of the children in this cohort.
Regarding the frequency of imaging tests, we found no changes in the prospective cohort after the publication of the NCD, probably as a consequence of the good diagnostic approach already used in the retrospective cohort. The high percentage of MRI performed in both cohorts is in line with the diagnostic recommendations of clinical practice guidelines, as has been reported in other series in Spain.4
The increasing trend in the performance of BC on admission was maintained in the prospective cohort, with a percentage of cases in which BC was performed similar to that of other studies 8 and a similar proportion of microbiological isolation from BC, which continues to be very low.2,8,14 In agreement with the previous literature,2,6,8,15 MSSA continued to be the main etiological agent of SD in children. The application of more sensitive microbiological techniques, such as polymerase chain reaction, may achieve an increase in the rate of microbial diagnosis2,8 and yield a more accurate identification of the microorganisms responsible for these infections, which would make it possible to corroborate whether the main role attributed to Kingella kingae in young children in other studies16–18 is maintained in Spain. The higher yield of culture of bone biopsy specimens, which ranges between 20% and 40% depending on the consulted series,2,8,14 was not observed in our study, since this technique is only performed per protocol in one of the hospitals. Although some recent guidelines recommend its performance to optimize the cost-effectiveness of microbiological testing in AOM,7 especially in areas with a higher prevalence of methicillin-resistant S. aureus strains, such as the United States, it is not an accessible technique for all centres. The need to perform it under sedation and to have an anaesthetic-surgical team available at diagnosis, combined with the favourable outcomes of these diseases with empirical antibiotic therapy, are probably responsible for the lack of implementation of this recommendation in routine clinical practice in our country.
The frequency of complications in our study was lower than the 20%–30% previously reported,15 with soft tissue abscesses as the main complication, which was consistent with the previous literature.4,15 The earlier diagnosis observed in our series could account for this low rate of complications, although due to the scarcity of microbiological isolates and the methodology of our study we were unable to determine whether the virulence of certain microorganisms or a potential severity bias could have affected the results in this regard.
In the analysis of the prospective cohort, we found that the NCD had an impact on the choice of empirical antibiotic therapy, both intravenous and oral, with a decrease in the use of combined intravenous regimens and of amoxicillin-clavulanic acid in favour of monotherapy with narrower-spectrum antibiotics, such as cefuroxime, as recommended in the document.11 The use of amoxicillin-clavulanic acid for empirical oral treatment was also reduced in favour of cephalosporins with a narrower antimicrobial spectrum and better tolerability, such as cefadroxil and cefuroxime-axetil. This reduction in the antibiotic spectrum did not lead to an increase in complications or sequelae in the prospective cohort, which, together with the lack of isolation of resistant microorganisms, justifies the maintenance in Spain of the recommendation to use monotherapy with narrower-spectrum beta-lactams offering coverage against MSSA and other pathogens involved in the development of PSD in patients older than 3 months (K. kingae and S. pyogenes),7 as stated in the NCD on the management of AOIs.11
The 4-day reduction in the duration of intravenous treatment and the 5-day reduction in the length of stay in the prospective cohort, both of which currently average 11 days, are encouraging findings in the new approach to these infections since the publication of the NCD, given the potential negative effects, both physical and psychological, that a prolonged hospital stay can have on children, in addition to a minor potential increase in antibiotic resistance. Despite this, the duration of intravenous antibiotic therapy in both cohorts is still far from the recommended short courses3,11,19 and therefore is a point of improvement in the management of these conditions to address in upcoming years while ensuring that there is no increase in complications or sequelae. To this end, it is essential to maintain active multicentre networks, such as RIOPed, in our case. In addition, the shorter duration of intravenous treatment was not accompanied by a reduction in the total duration of treatment due to the prolongation of outpatient oral antibiotherapy in the prospective cohort compared to the retrospective cohort, a longer duration that could not be attributed to a greater complexity of PSD in the prospective cohort. The total duration of treatment for PSD reported in other series is not less than 4–6 weeks,4,14,20,21 consistent with the mean duration of 5 weeks found in the retrospective cohort. The absence of further complications or sequelae in this cohort and the adequate outcomes of hematogenous OM involving nonvertebral locations with a total duration of treatment of 3–4 weeks compels us to insist on reducing the duration of antibiotherapy in uncomplicated PSD to 4 weeks2–4,6,7,15 and to evaluate the impact of this change in subsequent studies.
Considering that we performed a multicentre study including a large number of patients in whom the approach is becoming more standardized after publication of the NCD, the low rate of complications and sequelae observed could be due to the optimization of the diagnostic and therapeutic approach in the prospective cohort.
Our study has several limitations. The first is the relatively small sample size due to the low frequency of this disease, the fundamentally clinical nature of the diagnosis (although supported by imaging tests) and the scarcity of microbiological isolations. Nevertheless, it was a multicentre study that applied uniform recruitment criteria and with a duration of follow-up that is among the longest in the current literature.
In conclusion, in our series, PSD had a favourable outcome with a low frequency of complications (18.9%) and sequelae (8.6%). The most commonly isolated pathogen, although with a low frequency, was MSSA. Overall, the degree of adherence to the recommendations of the NCD for the management of paediatric AOI was good, with improvement compared to the retrospective cohort, and the recommendation to use MRI as the imaging test of choice due to its greater sensitivity for detection of soft tissue involvement and other associated complications should be maintained. Shorter antibiotic therapy regimens could be implemented in order to analyse the changes that occur in the population in this registry at future time points and to determine the need for new recommendations regarding the duration of treatment. Given the continuity of the RIOPed network, it will be possible to conduct studies in larger samples, which could help identify the factors associated with the development of complications and sequelae in children with PSD.
Conflict of interestThe authors declare that they have no conflict of interest.
We thank Maria Repice for her help with the English version of the text and the Sociedad Española de Reumatología Pediátrica (SERPE, Spanish Society of Paediatric Rheumatology) for the research grant that made it possible to launch the RIOPed network.
Velasco Arnaiz Eneritz MD (Hospital Sant Joan de Dèu, Barcelona, Spain), García-Fontecha César MD (Hospital Sant Joan de Dèu, Barcelona, Spain), Bustillo Alonso Matilde MD (Hospital Miguel Servet, Zaragoza, Spain), García Pardos Carmen MD (Hospital Universitario de Donosti, San Sebastián, Spain), Lirola Cruz María José MD (Hospital Sagrado Corazón, Seville, Spain), Díaz Delgado Rafael MD (Hospital Severo Ochoa, Madrid, Spain), Tagarro García Alfredo MD (Hospital Infanta Sofía, Madrid, Spain), Melendo Pérez Susana MD (Hospital Vall d’Hebrón, Barcelona, Spain), Domènech Marçal Elia MD (Hospital Germans Trias i Pujol, Barcelona, Spain), Martínez Campos Leticia MD (Hospital La Inmaculada, Almería, Spain), Menasalvas Ana MD (Hospital Virgen de la Arrixaca, Murcia, Spain), Guarch-Ibáñez Borja MD (Hospital Josep Trueta, Girona, Spain), Sanz Santaeufemia Francisco José MD (Hospital Niño Jesús, Madrid, Spain), Figueroa Ospina Lucía MD (Hospital de Villalba, Madrid, Spain), Camacho Lovillo Marisol MD (Hospital Virgen del Rocío, Seville, Spain), Pareja León Marta MD (Hospital General de Albacete, Spain), Gavilán Martín César MD (Hospital San Juan, Alicante, Spain), García Mazarío María Jesús MD (Hospital Universitario de Guadalajara, Spain), Couceiro Giranzo Jose MD (Hospital provincial de Pontevedra, Spain), Rivero-Calle Irene MD (Hospital Clínico de Santiago de Compostela, A Coruña, Spain), Pujol Soler Berta (Hospital General de Granollers, Barcelona, Spain), Bustabad Sagrario MD (Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain), García Alfaro María Dolores MD (Hospital Marqués de Valdecilla, Santander, Spain).