Since the first successful palliation was performed by Norwood et al. in 1983, there have been substantial changes in diagnosis, management, and outcomes of hypoplastic left heart syndrome, Survival for stage 1 palliation has increased to 90% in many centres, with patients potentially surviving into adulthood. However, the associated morbidity and mortality remain substantial.
Although the principles of staged surgical palliation of hypoplastic left heart syndrome are well established, there is significant variability in surgical procedure and management between centres, and several controversial aspects remain unresolved. In this review, we summarize the current surgical and management options for newborns with hypoplastic left heart syndrome and their outcomes.
Desde que Norwood et al. efectuaron la primera paliación exitosa en 1983, ha habido cambios sustanciales en el diagnóstico, manejo y pronóstico del síndrome de corazón izquierdo hipoplásico. La supervivencia en el estadio I de la paliación quirúrgica ha aumentado hasta el 90% en muchas instituciones, con la posibilidad de sobrevivir hasta la vida adulta. No obstante, la morbimortalidad asociada continúa siendo sustancial.
Aunque la premisa de la paliación quirúrgica por etapas del síndrome de corazón izquierdo hipoplásico está bien establecida, hay variaciones significativas en la técnica quirúrgica y el manejo entre distintos centros, y varios aspectos controvertidos siguen sin resolverse. En esta revisión resumimos las opciones quirúrgicas y de manejo disponibles actualmente para neonatos con síndrome de corazón hipoplásico, así como sus resultados.
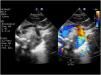
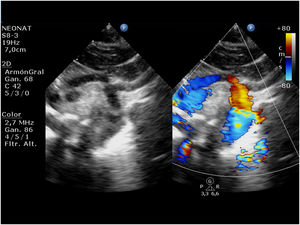
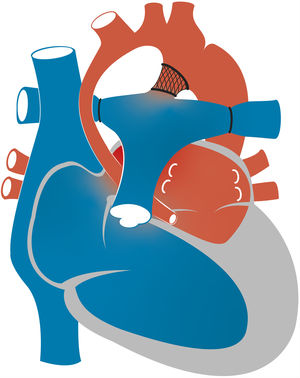
Hypoplastic left heart syndrome (HLHS) occurs in 0.16 to 0.36 per 1000 live births and accounts for 1.4%–3.8% of congenital heart defects. It is responsible for 23% of all cardiac deaths occurring in the first week of life.1 The term HLHS is used to cover a wide spectrum of lesions characterized by underdevelopment of the left heart structures, which in extreme cases manifest with aortic and/or mitral atresia or severe stenosis and hypoplasia or virtual absence of the left ventricle (LV) (Fig. 1).
In the 1970s, a multitude of articles in the surgical literature described various ingenious procedures that could allow survival of neonates with underdevelopment of left heart structures. However, there were no reports of successful stage 1 palliation (S1P) leading to a Fontan completion until William I. Norwood and colleagues described performance of an anastomosis connecting the proximal main pulmonary artery (PA) and the aorta at Boston Children’s Hospital in 1983.2 This revolutionary surgery using the right ventricle (RV) as the main pumping chamber for the systemic circulation became known as the Norwood procedure. In subsequent operations, the systemic and pulmonary circulations are separated by connecting the venae cavae directly to the pulmonary vasculature, also known as Fontan physiology.
Several technical and management modifications have been introduced overtime and have led to an increased survival of S1P to up to 90% in many institutions,3 but there are still controversial aspects and substantial peri-S1P and interstage morbidity and mortality.4 In this review, we describe the currently available surgical options for newborns with HLHS and their outcomes.
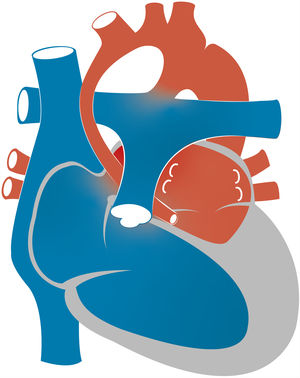
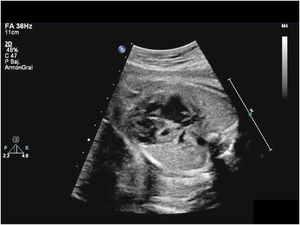
2Pathophysiology, clinical presentation and preoperative managementDuring intrauterine life, in which the physiological pulmonary vascular resistance (PVR) of the foetus is high and the systemic vascular resistance (SVR) low, the RV functions as the systemic ventricle and HLHS is well tolerated. Affected foetuses are usually born at term with adequate birth weights. Moreover, in the first hours of life there is a “honeymoon period” with minimal or no symptoms of haemodynamic instability. In this transitional situation, after the pulmonary venous return reaches the left atrium, while a small proportion of blood may reach the hypoplastic LV through the mitral valve, most of it enters the right atrium through a defect in the interatrial septum. Thus, oxygenated blood from the pulmonary veins is mixed with deoxygenated blood from the systemic venous return in the right atrium and then pumped to the RV and PA. From the pulmonary trunk, there are 3 “outlets” for the blood flow: the PAs and the ductus arteriosus (DA). In the absence of anatomical obstruction, the distribution of the flow depends on the relationship between the PVR and the SVR. Through the DA, the blood reaches the systemic circulation going into the descending aorta and retrogradely to the supra-aortic branches and coronary arteries (Fig. 2). There may also be a marginal anterograde flow contribution from the hypoplastic LV to the systemic circulation.
Closure of the DA and the resulting fall in the PVR leads to systemic hypoperfusion and pulmonary overcirculation, which rapidly progresses to haemodynamic shock. Therefore, the initial management of newborns with HLHS requires maintenance of ductal permeability with prostaglandins, balancing the systemic and pulmonary circulations mainly thorough manipulation of the PVR and ensuring adequate mixing at the atrial level. In the ideal preoperative situation, blood flow from the RV is distributed equally between both the pulmonary and the systemic circulations, maximizing oxygen delivery to the tissues and minimizing cardiac work. Preoperative stabilization of critically ill HLHS neonates with ECMO has also been described.5 Nevertheless, the need for mechanical circulatory support before or after the Norwood operation remains a major risk factor for mortality.6
The specific perioperative management of these patients is beyond the scope of this manuscript. In Spain, these complex patients are referred to one of six specific hospitals across the country accredited by The Ministry of Health as reference centres, departments and units, which include ours.
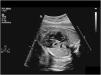
3Prenatal diagnosis and interventionAt present, many infants with HLHS receive a prenatal diagnosis, which allows planning of delivery and treatment (Fig. 3). The impact of prenatal diagnosis on surgical outcomes remains controversial. Most reports do not show a reduction in mortality with prenatal diagnosis.7,8 However, there is consistent evidence on improvements in morbidity following prenatal diagnosis of HLHS, including lower lactate levels and a better preoperative renal function, as well as a reduction in post-S1P seizures.7,9 The ability to detect the evolving hypoplastic LV (critical aortic stenosis) in mid-gestation makes it possible to perform aortic valvuloplasty in select foetuses. In 1991, Maxwell et al described their experience with dilatation of the aortic valve in 2 foetuses with severe aortic stenosis.10 Since this report, an increasing number of hospitals are attempting foetal cardiac interventions worldwide, and an International Fetal Cardiac Interventions Registry was established in 2010.11 In 2014, the foetal cardic intervention team at Boston Children’s Hospital reported the initial postnatal outcomes and survival in 100 patients that underwent foetal aortic valvuloplasty, of who 88 survived to birth and 38 achieved a biventricular circulation.12
Presence of an intact or highly restrictive intact atrial septum is associated with a high mortality. The mortality for S1P in patients with HLHS with a prenatal diagnosis of restrictive atrial septum is of approximately 50%.13 Attempts to open the atrial septum in utero have been associated with no change in outcome. This is in part due to the inability to ensure maintenance of the patency of the septum even after septoplasty.14
4Stage 1 of palliation: the Norwood procedure4aWith a modified Blalock-Taussig (MBT) shuntIn the classic Norwood procedure, pulmonary blood flow is provided by a MBT shunt which directs systemic flow from the innominate or subclavian artery to the right PA through a polytetrafluoroethylene (PTFE) shunt (Fig. 4). Due to the lower PVR relative to the SVR, there is continuous forward flow through the shunt, which results in a lower systemic diastolic blood pressure. “Coronary steal”, defined as decreased myocardial perfusion due to diastolic flow runoff into the pulmonary circulation, may develop in this context and potentially result in myocardial dysfunction, malignant arrythmias or sudden cardiac death. The other significant problem encountered with the MBT shunt is shunt stenosis and thrombosis. Various anticoagulant strategies and changes in material design and geometry in the MBT shunt may reduce the incidence of thrombosis. A recent systematic review of the Norwood procedure, mostly with a MBT shunt, found a reported incidence of thrombosis that ranged between 0% and 40%, while thromboembolic events (stroke or pulmonary embolisms) were rare. Although most studies involved long-term acetylsalicylic acid use, thromboprophylaxis strategies varied across centres.15
4bWith a right ventricle-to-pulmonary artery (RVPA) shuntEarly in the development of the Norwood procedure, valved and nonvalved RVPA shunts were used to establish pulmonary blood flow, but patients died within hours after surgery due to either pulmonary overcirculation or RV failure.16 Consequently, the RVPA shunt was abandoned in favour of the MBT shunt. However, in late 1990s Dr Shunji Sano repopularised the use of RVPA shunts to prevent the diastolic runoff and coronary steal associated with the MBT shunt (Fig. 4). In 2004, Sano reported the cases pf 33 consecutive patients with HLHS that underwent S1P surgery with placement of a nonvalved PTFE RVPA shunt. The early survival rate reached 89% However, there was an increased incidence of RV systolic dysfunction, arrhythmias and right ventricular volume overload.17 This was attributed to the need to perform right ventriculotomy to place the RVPA shunt, with its associated risks of myocardial dysfunction and arrhythmia, and to the nonvalved nature of the conduit, associated with regurgitation and RV volume overload.
4cMBT shunt versus RVPA shuntIn 2011, the Boston Children’s Hospital compared the medium-term outcomes of the MBT shunt versus the RVPA shunt in 118 HLHS patients that had undergone the Fontan operation.18 Of those patients, 36 had a RVPA shunt and 82 a MBT shunt after S1P. All patients but one survived the Fontan surgery. At the medium-term follow-up evaluation, outcomes and haemodynamic variables were similar between groups. However, more patients in the MBT shunt group exhibited tricuspid regurgitation, while patients in the RVPA shunt group require more PA catheter interventions. There were no significant differences between groups in RV function.
With the purpose of improving the limitations of the RVPA shunt, some technical modifications have been attempted in the S1P surgery: (1) one includes the use of a valved-RVPA shunt. However, a study that compared this intervention to the MBT shunt did not find any advantage in survival. Moreover, there was an increased need for subsequent conduit intervention19; (2) more recent approaches involve the insertion of a reinforced PTFE tube to prevent kinking and minimize myocardial injury. In this “dunking” technique, a small incision is made in the myocardium and dilated to accommodate the tube without resection of muscle. The ring-reinforced tube extends into the lumen of the RV. This new modification has been described in 39 patients and compared to a standard RVPA shunt in 48 patients. Patients with a ring-reinforced shunt had a lower frequency of subsequent interventions as well as higher aortic pulse pressures and improved PA growth up to the 1-year follow-up20 (see intraoperative images in Figs. 5 and 6); (3) placement of the RVPA shunt to the right or left of the aorta has been linked to improved outcomes in this population. A study from the Birmingham group evaluated 153 patients undergoing a S1P with either a right-sided (n = 125) or a left-sided (n = 28) RVPAS. There was evidence of lower cardiopulmonary bypass times, larger PA branches and a significant benefit in survival in the right-sided-group.21 A study that analysed data from the Single Ventricle Reconstruction Trial (SVRT) obtained similar results.22
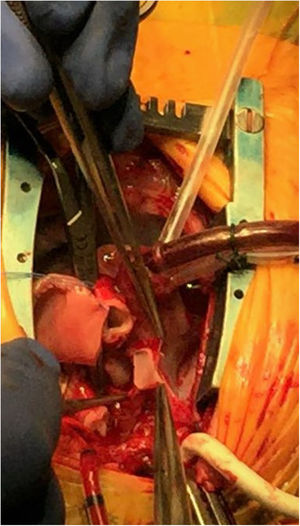
Intraoperative image of a Norwood surgery for stage I palliation in a hypoplastic left heart syndrome patient. At this point the ductal tissue has been resected, the coarctation repaired and the pulmonary artery augmented under selective cerebral and myocardial perfusion. In this image, the diminutive ascending aorta is opened down to the sinotubular junction and is ready to be anastomosed to the pulmonary artery. This part of the procedure is performed under selective cerebral perfusion.
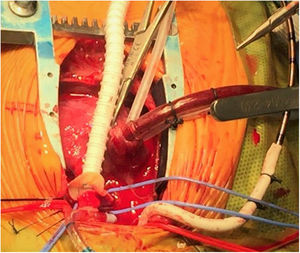
Intraoperative image of a Norwood surgery for stage I palliation in a hypoplastic left heart syndrome patient. An anastomosis between a ring-reinforced 5 mm shunt augmented with heterologous pericardium and the pulmonary artery bifurcation is being performed. The native pulmonary arteries are encircled with blue vessel loops. Note that the arterial inflow is provided through a 3.5 mm shunt anastomosed to the innominate artery while a single venous catheter is placed in the right atrium through its appendix. At this point the patient is being cooled and under cardiopulmonary bypass with continuous flow.
The widespread controversy over the potential risks and benefits of the MBT shunt versus the RVPA shunt motivated the development of the SVRT, which constituted a remarkable effort in the field of congenital heart surgery. Fifteen centres enrolled 555 patients that were randomized to S1P with either a MBT shunt or a RVPA shunt. The primary outcome was death or heart transplant (HT)-free survival at 12 months. Secondary outcomes included unplanned cardiovascular interventions, RV function, the hospital course and other serious adverse events.23 The RVPA shunt was found to be superior to the MBT shunt for the primary endpoint at 12 months (HT-free survival was 73.3% in patients with a RVPA shunt as compared to 63.3% in patients with a MBT shunt). There was a higher need for cardiopulmonary resuscitation during the S1P hospitalization in the MBT shunt group (20% vs 13%). However, unplanned interventions and complications were more common in patients with a RVPA shunt. In addition, PA growth and RV end diastolic volume and RV ejection fraction values were more favourable during follow-up in MBT shunt patients. The benefit in of the RVPA shunt compared to the MBT shunt decreased overtime, with no difference between groups after a mean follow-up of 32 months. These results have been validated by the follow-up data at 3 and 6 years. Patients with RVPA shunts required more catheter-based interventions, while patients in both groups suffered from serious complications overtime.24,25
4dAortic arch reconstructionNeoaortic arch obstruction is one of the key complications that affect morbidity and mortality after the S1P, with a current reported incidence of about 20% of patients. Arch obstruction can result in decreased cardiac output, RV dysfunction, and tricuspid regurgitation.3 Reconstruction of the aortic arch can affect neoaortic flow and thus coronary flow, too.
The first Norwood palliation achieved reconstruction of the aortic arch by direct anastomosis of the PA trunk and the diminutive aorta.2 Although this approach is promoted by some groups,26 it was abandoned by Norwood and others because the surgical anatomy was frequently deemed unsuitable for the procedure. In 2007, the Boston Children’s Hospital published data for 210 patients with that underwent S1P: 12 (6%) patients had a direct connection, 115 (55%) patients had an aortic homograft, 53 (25%) patients had a pulmonary homograft, and 30 (14%) patients had autologous pericardium interposition graft.3 Patients in whom the aortic arch was enlarged with autologous tissue were less likely to require intervention for management of neoaortic obstruction compared with those having homograft patch reconstruction. Furthermore, excision of all of the ductal tissue by means of coarctectomy reduced the risk of recurrent obstruction. More recent studies have confirmed the advantages of autologous tissue for aortic arch reconstruction, with a lower incidence of recoarctation, bronchial compression and PA branch compression.27 Data from the SVRT shows that 97 out of 549 patients (18%) required interventional catheterization or surgical procedures for recoarctation, performed most frequently during pre-stage II intervention or concomitant with stage II surgery.28
4eTricuspid regurgitation and RV dysfunctionThe presence of significant RV dysfunction or tricuspid regurgitation (TR) have been consistently associated with the inability to progress through staged palliation as well as poor outcomes. Although tricuspid valve repair is successful in reducing regurgitation in most patients, its positive impact is hindered by the limited duration of the repair, with significant recurrent regurgitation developing in one-third of patients.29 Right ventricular dysfunction is progressive and a major determinant of HT-free survival. Furthermore, interstage mortality remains high in HLHS patients (12%) and seems to be related to the RV ejection fraction and TR, as well as additional factors such as preterm birth, aortic-and-mitral atresia subtype and socioeconomic status.4,30
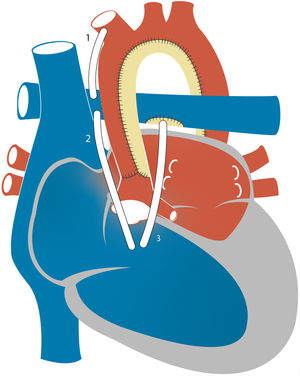
5Hybrid approachIn 1992, Gibbs and colleagues proposed stenting of the arterial duct for palliation, which they performed in 2 neonates with pulmonary atresia.31 One year later, Ruiz et al. used ductal stenting in 6 patients with HLHS as a bridge to HT.32 The same year, Gibbs et al. reported performance of ductal stenting and bilateral PA banding in 4 newborns with HLHS.33 This hybrid approach achieves the same goals as the Norwood procedure: the ductal stent establishes the systemic blood flow and bilateral banding restricts pulmonary blood flow, thus reducing the potential coronary steal. Cerebral and coronary perfusion is achieved through the aortic arch in a retrograde fashion, contrary to the surgical S1P (Fig. 7). Stenosis or obstruction of the connection between the descending aorta and aortic arch (retrograde aortic arch obstruction) is a significant complication that has been reported in up to 25% of patients.34 The use of a “reverse Blalock-Taussig shunt” from the main PA to the innominate artery has been proposed to avoid the complications of retrograde arch obstruction in hybrid palliation.35 Moreover, avoiding cardiopulmonary bypass could have important benefits in newborns. A recent comparison of 49 patients managed with the classic Norwood surgery and 13 with the hybrid procedure after completion of the Fontan surgery showed that patients treated with the hybrid procedure had poorer weight gain before establishing the superior cavopulmonary connection which, although recovered by Fontan completion, could be a risk factor for morbidity and poorer neurodevelopmental outcomes.36
Over the last decade, the hybrid approach has been applied to many patients with HLHS. Galantowicz et al. and more recently Yerebekan et al. have reported 90% and 97.5% survival in 40 and 182 patients with HLHS undergoing a hybrid procedure, respectively.37,38 Nevertheless, there is evidence that bilateral banding is associated with an increase in the number of branch PA interventions with a significant proportion of patients failing to progress through palliation at this point or after the comprehensive stage 2 procedure.38
A study from the Congenital Heart Surgeons Society evaluated hybrid procedures as an alternative to the classic S1P in 564 neonates managed with the Norwood operation with either a MBT shunt or RVPA shunt or a hybrid procedure. The risk-adjusted 4-year survival was better in patients managed with a RVPA shunt compared to the MBT shunt or the hybrid procedure (76% vs 60% vs 61%).39 A recent meta-analysis reported a lower early survival for the hybrid procedure compared to the classic S1P approach.40 Consequently, most centres currently rely on the surgical Norwood operation and reserve the use of the hybrid approach for the most fragile patients (low birth weight or restrictive atrial septum) or those presenting in shock.
6TransplantationDespite significant improvements in the outcomes of patients with HLHS, there is still substantial morbidity and mortality through staged palliation,30 and recent large care series suggest that the survival after the Norwood procedure has plateaued.41 A recent meta-analysis comparing the long-term outcomes of HLHS palliation with either a MBT shunt or an RVPA shunt found that the 1-, 4- and 6-year HT-free survivals for the MBT shunt were 67%, 64% and 63%, respectively, compared to 75%, 67%, and 61% for the RVPA shunt. Thus, the observed survival differed significantly between the 2 shunts was significant at 1 year but was comparable thereafter.42
The use of orthotopic HT for initial palliation of neonates with HLHS was first described by L. Bailey.43 This group reported the outcomes of HT in 28 neonates with HLHS between 1985 and 1989. Early mortality rate was 18%. However, an 89% operative survival was achieved in the last 19 patients of the series. An update on the pioneering Loma Linda University experience on 111 HLHS patients entering the transplant protocol showed a 5-year survival of 81%, which reflected recent improvements in anti-rejection protocols.44 Given the long waiting times for neonatal HT and the progressive improvements in the multistage palliation strategy, heart transplant is rarely offered as first-line treatment to HLHS patients at present. Attempts have also been made to increase the donor pool through the use of ABO-incompatible donors.
Transplantation is also offered to HLHS patients who fail to progress through staged palliation or develop complications of the Fontan circulation. An analysis of risk factors for being placed in the HT waitlist and associated outcomes after the Norwood procedure was made with data from the SVRT, in which 33 patients were listed and 18 underwent HT. The mortality was 39% in the waiting list and 33% after HT. Nevertheless, HT for rescue following the Norwood operation in the first year of life still carries a significant risk of death. Another study in 253 children that underwent HT after palliation with the Norwood procedure found that survival was not affected by last palliation stage or sensitization.45
7ConclusionsPrior to the original breakthrough described by William I. Norwood and colleagues in 1983, unoperated patients born with HLHS had almost no chance for survival. Since the first description of successful surgical palliation, there have been major improvements in the management of these patients, with many centres reporting survival rates of 90% for S1P. Moreover, completion of staged palliation and survival into adulthood is now possible. However, the long-term morbidity and mortality in HLHS patients remain disturbingly high. The outcomes of HT in both unoperated and operated patients have been improving. Nonetheless, just as important advances in medical and surgical care have improved outcomes for HLHS this far, we are confident that they will continue to improve the long-term outcomes of patients born with this challenging disease.
Conflict of interestThere is no conflict of interest to disclose.
We thank Ana L. Teruel Martínez for the illustrations in the figures of this articles.
Please cite this article as: Cómo citar este artículo: Bautista-Hernandez V, et al. Opciones quirúrgicas actuales y sus resultados en neonatos con síndrome de corazón izquierdo hipoplásico. An Pediatr (Barc). 2019;91:352.