We present the case of a boy aged 16 months who presented with a blue-black hemorrhagic plaque in the left parieto-occipital region extending to the eyelid and earlobe and had appeared eight hours before (Fig. 1A). There was no history of traumatic injury. Upper respiratory tract infection preceded its development. The patient was afebrile, in good general health, without hematomas, petechiae or ecchymosis in other locations. The salient laboratory findings were thrombocytopenia (49 000 platelets/μL) and severe coagulopathy with absence of coagulation (undetectable) and a D-dimer level of 976 mg/L. There were no signs of malignancy or hemolysis. The patient also had leukocytosis with neutrophilia and slight elevation of acute phase reactants. The chest radiograph revealed nonspecific bilateral perihilar infiltration. The examination of the lesion by Doppler ultrasound and cranial CT confirmed the presence of extra-axial cephalohematoma in absence of intracranial bleeding or thrombosis.
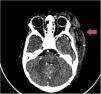
In light of these findings, the patient was treated with vitamin K, fibrinogen, fresh frozen plasma (FFC) and a platelet transfusion that achieved normalization of coagulation, in addition to antibiotherapy with cefotaxime and azithromycin. Diagnostic tests for infectious agents turned out negative, with the except of a positive PCR result for rhinovirus in a nasopharyngeal aspirate sample. At 24 h, follow-up imaging tests (CT angiography [Fig. 2] and magnetic resonance angiography) demonstrated that the lesion was stable. The etiological investigation was expanded, revealing normal coagulation factor levels, a direct Coombs test that was weakly positive for C3, and normal results for antithrombin III and ADAMTS-13. There was a significant reduction in protein C levels (PC, 28%) and protein S levels (6.5%). Suspicion of purpura fulminans associated with PS deficiency promoted initiation of treatment with immune globulin, steroids and FFC. At 48 h, the patient developed partial thrombosis of the right brachial vein associated with a peripherally inserted central catheter that was treated with heparin. Protein S levels increased progressively and had normalized at 48 h. Protein S levels were normal in the family study. The diagnosis was confirmed by a positive result for PS autoantibodies. Testing for factor V Leiden revealed that both the mother and the patient carried one copy of the mutation. Since the plaque became necrotic, the patient required surgical debridement at 3 weeks (Fig. 1B) and hyperbaric oxygen therapy in the months that followed to promote wound healing (Fig. 1C).
Idiopathic purpura fulminans (IPF) is a form of purpura fulminans caused by autoantibodies against PS.1 Proteins S and C are proteins primarily synthesized in the liver with anticoagulant activity dependent on endothelial vitamin K. Protein C inhibits the formation of blood clots, forming a complex with thrombin and thrombomodulin by binding the endothelial PC receptor, thus “switching off” their procoagulant activity. Protein acts as a cofactor of PC, inhibiting the coagulation cascade at the level of factors Va and VIIIa.2
The onset of IPF tends to occur 7–10 days after infection and results from cross-reactivity against the infectious agent and protein S via molecular mimicry, giving rise to PS autoantibodies.1,3 This disease has been described in association with infection by varicella-zoster virus, streptococcus or herpes simplex type 6.1,3,4 In the case of our patient, there was only a positive PCR result for rhinovirus in a nasopharyngeal aspirate sample.
The disease begins with the appearance of progressively enlarging purplish black areas of hemorrhagic cutaneous necrosis that can result in hemorrhagic infarction and formation of painful and indurated bullae.5 Unlike cases associated with meningococcal sepsis, which involve the distal extremities, IPF tends to involve the thighs. sparing the distal portion of the extremities, the glutes and, in male individuals, the penis and scrotum. Involvement of the upper body or extremities is infrequent, and involvement of the head and neck is extremely rare.1,3 In the case of our patient, it was limited to the face and earlobe.
Patients present in good general health, with no signs of sepsis, but with laboratory findings suggestive of disseminated intravascular coagulation.2 A characteristic feature is the isolated deficiency of PS and PC with normal coagulation factors. The detection of IgG antibodies against PS confirms the diagnosis.1 There have been reports of cases associated with inherited thrombophilia, with detection of Leiden V factor (as was the case of our patient), prothrombin gene polymorphisms or anticardiolipin antibodies.4 Treatment should be initiated as soon as IPF is suspected without awaiting confirmation. The treatment is based on correcting the deficiency and controlling the immune mechanism. Fresh frozen plasma should be administered at regular intervals until the coagulopathy resolves, with administration of prothrombin complex concentrate in refractory cases. Heparin should be added in the event of thrombotic complications.1,2 In terms of immunomodulation, the first choice is immunoglobulin infusion, followed by plasmapheresis if it fails.1 Surgery plays a key role in the management of complications, with procedures such as debridement of necrotic areas or early fasciotomy to avoid amputation.6 No cases of recurrence have been reported. In the case of our patient, with involvement of an area of the body not yet reported in the literature in which potential complications could be fatal, steroids were added to the immunoglobulin infusion, and the patient did not require plasmapheresis at any point. He underwent surgical debridement and required hyperbaric oxygen therapy.