In Spain, the tools to diagnose COVID-19 were available in primary care from May 2020. Previously most studies described inpatients or patients in A&E departments, and fever and cough were the most frequent symptoms. This study aims to define the clinical picture of the pediatric COVID-19 in the community.
MethodsA descriptive and analytical observational study was performed including pediatric cases (0−14years) from 255 pediatricians, proportionally distributed to its population, from primary health centers in Spain, from 12th May 2020 to 30th April 2021. Diagnostics were made by PCR detection of viral RNA, rapid antigen detection test or positive IgG serology.
ResultsThere were 10,021 positive children included, 48.4% women, mean age 8,04±4.17years. Infection was detected due to contact tracing (70.9%), compatible symptoms (18.8%). Household was the main source of transmission (64.9%), followed by school setting (10%) or unknown (9.9%). We did not find any significant differences in the incidence between holidays and school terms. 43.2% of the children were asymptomatic. Most frequent symptoms are rhinorrhea in <2years, fever in 3−8years and headache in >9years. An exhaustive description of objective and subjective symptoms by age is made. 18 patients were hospitalized, one with multisystem inflammatory syndrome in children. There were no deaths.
Conclusionspediatric COVID-19 is a mild disease, with a large number of asymptomatic cases, with very few hospital admissions and deaths. The main setting for transmission is the household, and school closures should be a last resource measure during the COVID-19 pandemic. A specific clinical picture of pediatric COVID-19 was not found.
En España, las pruebas diagnósticas de COVID-19 en atención primaria estuvieron disponibles desde mayo de 2020. Previamente la mayoría de los estudios describían pacientes hospitalarios: la fiebre y la tos eran los síntomas más frecuentes. Interesaba conocer la expresión clínica de la COVID-19 pediátrica en la comunidad.
Métodosestudio descriptivo analítico observacional de casos pediátricos (0−14 años) de 255 pediatras de atención primaria españoles, del 12/5/2020al 30/4/2021. Los diagnósticos se determinaron por PCR, test rápido de detección de antígeno o serología IgG positiva.
ResultadosSe incluyeron 10 021 niños, 48,4% mujeres, con una edad media de 8,04±4,17 años. Se detectó la infección por búsqueda de contactos (70,9%) o síntomas compatibles (18,8%). El hogar familiar fue la principal fuente de contagio (64,9%), seguido por los colegios (10%) o de origen desconocido (9,9%). No hubo diferencias significativas en la incidencia entre temporadas vacacionales o lectivas. El 43,2% fueron asintomáticos. Los síntomas más frecuentemente encontrados fueron rinorrea en menores de 2 años, fiebre entre 3 y 8 años y cefalea en mayores de 9 años. Se describen exhaustivamente los síntomas y signos observados por edad. Se hospitalizaron 18 pacientes, uno con un síndrome inflamatorio multisistémico. No hubo fallecimientos.
ConclusionesLa COVID-19 es una enfermedad leve con un gran número de casos asintomáticos, con pocas hospitalizaciones y fallecimientos. El lugar principal de transmisión es el domicilio y el cierre de colegios debería ser el último recurso para controlar la pandemia. No se pudo describir un cuadro clínico característico de la enfermedad.
On January 12, 2020, a novel coronavirus was identified that has come to be known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), while the associated disease is known as coronavirus disease 2019 (COVID-19).1 Although the clinical picture of COVID-19 in patients that require hospitalization has been characterized in detail, there are few descriptions of the clinical presentation in paediatric cases in the community. As of October 6, 2021, the National Epidemiological Surveillance Network of Spain2 had registered a much lesser number of hospital admissions in individuals aged less than 19 years compared to all other age groups (1.974% of children aged less than 5 years, 0.450% of those aged 5–9 years and 0.606% of those aged 10–19 years), as well as a lesser number of admissions to the intensive care unit (ICU) (0.057, 0.014 and 0.031% in the aforementioned age groups, respectively) and of deaths (0.005, 0.004 and 0.001%, respectively), in agreement to the findings in other countries.3
The clinical cases in children and adolescents described in the literature initially referred to those who had been hospitalised or visited the emergency department, and cough and fever were the most common presenting symptoms.4 These symptoms are nonspecific in paediatric patients, who tend to experience 4–8 episodes of respiratory infection per year. Published studies underestimated the frequency of asymptomatic and oligosymptomatic patients, as they were conducted in hospital settings, where patients seek care for more intense, severe or persistent symptoms.5,6 Knowledge of the symptoms experienced in the community can guide the ordering of active infection diagnostic tests (AIDTs).
Although the presence of risk factors, such as type 1 diabetes or congenital heart disease, is associated with a higher rate of admission,7 and chronic pulmonary disease, obesity, neurologic or neurodevelopmental disorders and cardiovascular disease are associated with a higher mortality,8 it would also be interesting to know whether these comorbidities are associated with an increased risk of developing the disease. In addition, a multisystemic inflammatory syndrome in children (MIS-C) has been described in association with SARS-COV-2, a rare and severe complication with predominance of gastrointestinal symptoms predominate over respiratory ones.9
As AIDTs became available for contact investigation, it became apparent that the probability of developing symptoms or severe disease was lower in young individuals compared to adults.10,11 And while young individuals acquired the infection as readily as the rest of the population, they did not appear to be the main spreaders of the disease.12–14
From May 11, 2020, AIDTs became widely available in Spain, both in the hospital and primary care settings. Primary care paediatricians in Spain are integrated in a universal and free public health system and provide services to the vast majority of the paediatric population in the country.
In September 2020, we started a study with the aim of describing the main presenting signs and symptoms of COVID-19 in the paediatric population of Spain managed at the primary care level.
Material and methodsDesign: single-cohort retrospective and prospective longitudinal study. Setting: Spain. Period under study: we collected retrospective data on positive patients for the period ranging from May 12 to December 1, 2020. We collected data prospectively between December 1, 2020 to April 30, 2021. Sample: Study universe: paediatric population of Spain (0−14 years). Inclusion criteria: every child included in the caseloads of collaborating paediatricians given a diagnosis of SARS-CoV-2 infection based on positive results of the polymerase chain reaction (PCR) test, rapid antigen test (RAT) and/or IgG antibodies test. Exclusion criteria: previous positive PCR test for SARS-CoV-2 (if a child had 2 or more positive results, we only included the first one in the analysis); clinical diagnosis of infection not confirmed by diagnostic tests; and retrospective cases for which there was not sufficient information. Sample size calculation: for an unlimited population size, a level of confidence of 0.95 and a precision of 0.05, taking as reference the most frequently reported symptoms of infection by SARS-CoV-2 in paediatrics (cough, with a relative frequency of 48.1%),15 we estimated that we needed a minimum of 384 patients.
Data collection. We collected information through the online database of the paediatric primary care surveillance network of Spain (Red de Vigilancia en Pediatría de Atención Primaria [PAPenRED]), which includes 318 primary care paediatricians distributed evenly throughout the Spanish territory.
Each collaborating paediatrician selected all children in the corresponding caseload with diagnosis of SARS-CoV-2 infection confirmed by PCR, RAT and/or antibody testing. The selection of the type of test was based on the judgment of the paediatrician and availability. We included cases diagnosed from 12 May, 2020 (date when the PCR test became widely available) and December 1, 2020 in any care setting (primary care, urgent care, emergency care and inpatient care in both public and private hospitals) retrospectively, and patients diagnosed between December 2020 and April 2021 whose parents or legal guardians signed the informed consent form prospectively. We collected data for the 15 days that followed diagnosis.
We defined suspected case as any patient with symptoms of acute respiratory tract infection of sudden onset and any severity presenting with fever, cough or shortness of breath. Odynophagia. Anosmia, ageusia, myalgia, diarrhoea, chest pain or headache, among others, were also considered symptoms that supported the suspicion. We also included asymptomatic cases detected through contact tracing or population screening.
Variables. Patient-related: age, sex and risk factors. SARS-CoV-2 infection-related: date of diagnosis, type of diagnostic tests performed, reason for testing and source of transmission (if known). In symptomatic patients, we documented the time elapsed from onset to testing. Symptom-related: objective symptoms: cough, rhinorrhoea, fever, irritability, vomiting, diarrhoea and exanthema. Subjective symptoms (in patients aged more than 4 years): myalgia, abdominal pain, headache, odynophagia, anosmia/dysgeusia and general malaise. In symptomatic patients, each paediatrician noted the disease they suspected based on the symptoms (upper respiratory tract infection, flu-like illness, bronchospasm, pneumonia, pharyngitis, fever without source, laryngitis, gastroenteritis, viral exanthema or exanthema of unknown aetiology). Outcomes: patient outcome and total duration of symptoms.
Statistical analysis. We performed a descriptive analysis of the variables: for continuous variables, we used the mean and standard deviation (SD), and for categorical variables, absolute and relative frequencies (percentages with the corresponding 95% confidence intervals [CIs]).
We measured the frequency of asymptomatic patients and the frequency of each symptom in relation to age and duration of symptoms. To compare proportions, we calculated odds ratios (ORs) with the corresponding 95% CIs, and to compare means we calculated mean differences with the corresponding 95% CIs. We defined statistical significance as a P value of less than 0.05.
The statistical analysis was performed with the software IBM SPSS Statistics, version 25.0. (IBM Corp; Armonk, NY, USA).
Ethical and legal considerations: the study adhered to the International Conference On Harmonisation (ICH) Harmonised Tripartite Guideline For Good Clinical Practice (Law 14/2007 of Biomedical Research) and the ethical principles of the Declaration of Helsinki. The study was approved by the Clinical Research Ethics Committee of Huelva under file 09/20 (05/11/2020.
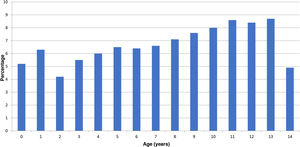
ResultsA total of 255 paediatricians collaborated in the study. The initial sample included 10 101 positive cases, of which 10 021 (99.2%) were valid for the analysis. Of all patients in the study, 48.4% (95% CI, 47.4–49.4) were female. The mean age was 8.04 years (SD, 4.17). Fig. 1 presents a histogram of the case distribution by year. In most regions in Spain, paediatricians care for children until age 15 years, although there are some where children are transferred to general medicine on their 14th birthday. In 87.9% of the patients, there was no underlying disease that posed a risk for severe disease, while 773 patients (7.7%) had asthma or another pulmonary disease, 400 (4.0%) obesity, 65 (0.6%) heart disease, 30 (0.3%) were immunosuppressed and 18 (0.2%) had other risk factors. In the subset of patients with asthma, 81.4% had mild asthma; 17.3% moderate asthma and 1.3% severe asthma.
The test was performed on account of close contact with a case in 70.9% of patients (95% CI, 70.0–71.8), of compatible symptoms in 18.8% (95% CI, 18.0–19.6) and on account of both contact and symptoms in 9.5% (95% CI, 8.9–10.1). The rest of the tests were performed in the context of a screening programme (0.6%; 95% CI, 0.4−0.8) or an epidemiological study (0.2%; 95% CI, 0.1−0.3).
The source of transmission was a household member in 69.4% of cases (95% CI, 68.5–70.3), and this was the most frequent source of transmission in every age. In 10.0% of cases (95% CI, 9.4–10.6), transmission occurred in the school or childcare centre. In 7.6% (95% CI, 7.0–8.1), it occurred through caregivers or relatives that did not live in the household, and in 2.6% (95% CI, 2.3–2.9) through other social interactions. The source of transmission was not identified in 9.9% of cases. (95% CI, 9.3–10.5). During holiday periods, transmission in childcare centres declined drastically to 4.7%, while the other sources of transmission remained at similar levels. We ought to highlight that in the subset of patients who underwent testing on account of compatible symptoms alone (18.8% of the total), the source of transmission was not identified in 49.3% of cases.
The positive diagnostic tests consisted of 7482 PCR tests (74.7%), 2582 RATs (25.8%) and 231 IgG antibody tests (2.3%). The diagnosis was made with a single test in 9079 patients (90.6%). Two tests were necessary in 695 cases (6.9%) and 3 or 4 in 247 (2.5%). In 497 children (5%) the initial RAT was negative but, due to persistence of symptoms, a PCR test (in 95.6% of them) or antibody test (remaining 4.4%) was performed at a later time. Conversely, 110 children (1.1%) who had a negative initial PCR test turned out positive in a subsequent RAT (60%) or PCR test (40%).
The mean time elapsed from onset to performance of the diagnostic test was 1.93 days (SD, 2.2; median, 1 day). By type of test, PCR tests were performed in a mean of 2.0 days (SD, 2.3), RAT in 1.6 days (SD, 1.6) and IgM and IgG tests in 3.8 days (SD, 3.8).
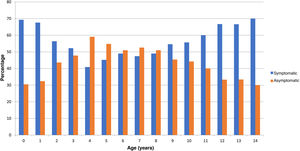
In the checkup performed at 15 days post onset, 4330 patients were asymptomatic, which amounts to 43.2% of the total (95% CI, 42.2–44.1). Fig. 2 shows the distribution of cases by age. A higher proportion of infants and adolescents developed symptoms, and asymptomatic cases were more frequent in intermediate ages. Table 1 presents the odds ratios for developing symptoms during the infection by sex, age and risk factors, and we found a significant association with the presence of pulmonary disease (including asthma) and obesity. Having moderate or severe asthma, compared to mild asthma, was not associated with an increase in symptoms, with an OR of 1.24 (95% CI, 0.84–1.83).
Odds ratio for being symptomatic based on the variables under study.
| Odds Ratio | 95% CI* | |
|---|---|---|
| Sex | ||
| Male/female | 1.00 | 0.93−1.09 |
| Age | ||
| <1 | Reference | Reference |
| 2−3 | 0.75 | 0.60−0.94 |
| 4−6 | 0.39 | 0.32−0.47 |
| 7−10 | 0.48 | 0.39−0.58 |
| 11−14 | 0.83 | 0.68−1.01 |
| Presence of risk factors | ||
| Yes/No | 1.31 | 1.16−1.49 |
| Immunosuppression | ||
| Yes/No | 1.41 | 0.55−2.37 |
| Heart disease | ||
| Yes/No | 1.14 | 0.69−1.88 |
| Pulmonary disease (including asthma) | ||
| Yes/No | 1.31 | 1.13−1.53 |
| Obesity | ||
| Yes/No | 1.41 | 1.14−1.74 |
Table 2 presents the frequency of objective symptoms by age, and Table 3 the frequency of subjective symptoms by age. The most frequent symptoms observed in children with SARS-CoV-2 infection were fever (2641 cases; 23%), rhinorrhoea (2487; 23%) and cough (2231; 22%). Other frequent symptoms were headache (1929; 44%), general malaise (1200; 27.4%) and odynophagia (1061; 24%). There were also patients with anosmia (668; 15%) and gastrointestinal symptoms, including diarrhoea (821; 14%), abdominal pain (619; 14%) and vomiting (312; 5%). By age group, the most frequent symptom in children up to age 2 years was rhinorrhoea (505/786; 64%), in children aged 2–8 years, fever (684/1458; 47%) and in children aged 8–14 years, headache (1601/3209; 50%). In the subset of patients with fever, 43.9% developed low-grade fever, 46.3% a temperature between 38 and 39°C and 5.7% a temperature greater than 39°C. High fever was more frequent in the first 3 years of life. The most frequent working diagnoses initially made by paediatricians was “upper respiratory tract infection” (n=2296; 43%; 95% CI 42.1–44.1) or “uncertain” (n=1.340, 25.2%, 95% CI 24.3–26.1%).
Objective (observable) symptoms by age.
| Age (years) | n | Cough | Rhinorrhoea | Fever | Irritability | Vomiting | Diarrhoea | Exanthema | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | ||
| <1 | 361 | 190 | 52.6 (47.5−7.8) | 224 | 62.0 (57.0−67.1) | 220 | 60.9 (55.9−0.66) | 94 | 26.0 (21.5−30.6) | 19 | 5.3 (3.0−7.6) | 82 | 22.7 (18.4−27.0) | 13 | 3.6 (1.7−5.5) |
| 1 | 425 | 248 | 58.4 (53.7−63.0) | 281 | 66.1 (61.6−70.6) | 241 | 56.7 (52.0−61.4) | 68 | 16.0 (12.5−19.5) | 38 | 8.9 (6.2−11.7) | 94 | 22.1 (18.2−26.1) | 27 | 6.4 (4.0−8.7) |
| 2 | 238 | 135 | 56.7 (50.4−63.0) | 133 | 55.9 (49.6−62.2) | 135 | 56.7 (50.4−63.0) | 22 | 9.2 (5.6−12.9) | 17 | 7.1 (3.9−10.4) | 56 | 23.5 (18.1−28.9) | 22 | 9.2 (5.6−12.9) |
| 3 | 289 | 142 | 49.1 (43.4−54.9) | 146 | 50.5 (44.8−56.3) | 149 | 51.6 (45.8−57.3) | 12 | 4.2 (1.9−6.5) | 14 | 4.8 (2.4−7.3) | 46 | 15.9 (11.7−20.1) | 12 | 4.2 (1.9−6.5) |
| 4 | 247 | 102 | 41.3 (35.2−47.4) | 99 | 40.1 (34.0−46.2) | 131 | 53.0 (46.8−59.3) | 7 | 2.8 (0.8−4.9) | 21 | 8.5 (5.0−12.0) | 41 | 16.6 (12.0−21.2) | 11 | 4.5 (1.9−6.5) |
| 5 | 294 | 114 | 38.8 (33.2−44.3) | 128 | 43.5 (37.9−49.2) | 135 | 45.9 (40.2−51.6) | 3 | 1.0 (0.1−2.2) | 16 | 5.4 (2.8−8.0) | 44 | 15.0 (10.9−19.0) | 10 | 3.4 (1.3−5.5) |
| 6 | 312 | 95 | 30.4 (25.3−35.6) | 122 | 39.1 (33.7−44.5) | 123 | 39.4 (34.0−44.8) | 4 | 1.3 (0.0−2.5) | 16 | 5.1 (2.7−7.6) | 29 | 9.3 (6.1−12.5) | 16 | 5.1 (2.7−7.6) |
| 7 | 316 | 107 | 33.9 (28.6−39.1) | 125 | 39.6 (34.2−44.9) | 146 | 46.2 (40.7−51.7) | 3 | 0.9 (0.0−2.0) | 22 | 7.0 (4.2−9.8) | 45 | 14.2 (10.4−18.1) | 16 | 5.1 (2.6−7.5) |
| 8 | 350 | 126 | 36.0 (31.0−41.0) | 111 | 31.7 (26.8−36.6) | 154 | 44.0 (38.8−49.2) | 2 | 0.6 (0.0−1.4) | 19 | 5.4 (3.1−7.8) | 54 | 15.4 (11.6−19.2) | 13 | 3.7 (1.7−5.7) |
| 9 | 418 | 121 | 28.9 (24.6−33.3) | 165 | 39.5 (34.8−44.2) | 169 | 40.4 (35.7−45.1) | 1 | 0.2 (0.0−0.7) | 25 | 6.0 (3.7−8.3) | 53 | 12.7 (9.5−15.9) | 6 | 1.3 (0.3−2.6) |
| 10 | 445 | 125 | 28.1 (23.9−32.3) | 167 | 37.5 (33.0−42.0) | 201 | 45.2 (40.5−49.8) | 2 | 0.4 (0.0−1.1) | 26 | 5.8 (3.7−8.0) | 52 | 11.7 (8.7−14.7) | 6 | 1.3 (0.3−2.4) |
| 11 | 515 | 173 | 33.6 (29.5−37.7) | 187 | 36.3 (32.2−40.5) | 220 | 42.7 (38.4−47.0) | 6 | 1.2 (0.2−2.1) | 23 | 4.5 (2.7−6.2) | 60 | 11.7 (8.9−14.4) | 13 | 2.5 (1.2−3.9) |
| 12 | 561 | 208 | 37.1 (33.1−41.1) | 227 | 40.5 (36.4−44.5) | 227 | 40.5 (36.4−44.5) | 9 | 1.6 (0.6−2.6) | 25 | 4.5 (2.7−6.2) | 73 | 13.0 (10.2−15.8) | 13 | 2.3 (1.1−3.6) |
| 13 | 577 | 215 | 37.3 (33.3−41.2) | 239 | 41.4 (37.4−5.4) | 244 | 42.3 (38.3−46.3) | 4 | 0.7 (0.0−1.4) | 23 | 4.0 (2.4−5.6) | 54 | 9.4 (7.0−11.7) | 16 | 2.8 (1.1−3.6) |
| 14 | 343 | 130 | 37.9 (32.8−43.0) | 133 | 38.8 (33.6−43.9) | 146 | 42.6 (37.3−47.8) | 5 | 1.5 (0.2−2.7) | 8 | 2.3 (2.4−5.6) | 38 | 11.1 (7.8−1.4) | 9 | 2.6 (0.9−4.3) |
| 0−14 | 5691 | 2.231 | 39.2 (37.9−40.5) | 2.487 | 43.7 (42.4−45.0) | 2.641 | 46.4 (45.1−47.7) | 242 | 4.3 (3.7−4.8) | 312 | 5.5 (4.9−6.1) | 821 | 14.4 (13.5−15.3) | 212 | 3.7 (3.2−4.2) |
Subjective or non-observable symptoms by age.
| Age (years) | n | Myalgia | Abdominal pain | Headache | Odynophagia | Anosmia | General malaise | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % | ||
| 4 | 247 | 15 | 6.1 (3.1−9.1) | 41 | 16.6 (12.0−21.2) | 47 | 19.0 (14.1−23.9) | 27 | 10.9 (7.0−14.8) | 5 | 2.0 (0.3−3.8) | 25 | 10.1 (6.4−13.9) |
| 5 | 294 | 23 | 7.8 (4.8−10.9) | 40 | 13.6 (9.7−17.5) | 70 | 23.8 (18.9−28.7) | 51 | 17.3 (13.0−21.7) | 11 | 3.7 (1.6−5.9) | 48 | 16.3 (12.1−20.6) |
| 6 | 312 | 32 | 10.3 (6.9−13.6) | 47 | 15.1 (11.1−19.0) | 99 | 31.7 (26.6−36.9) | 52 | 16.7 (12.5−20.8) | 18 | 5.8 (3.2−8.4) | 57 | 18.3 (14.0−22.6) |
| 7 | 316 | 38 | 12.0 (8.4−15.6) | 58 | 18.4 (14.1−22.6) | 112 | 35.4 (30.2−40.7) | 57 | 18.0 (13.8−22.3) | 24 | 7.6 (4.7−10.5) | 66 | 20.9 (16.4−25.4) |
| 8 | 350 | 40 | 11.4 (8.1−14.8) | 68 | 19.4 (15.3−23.6) | 153 | 43.7 (38.5−48.9) | 66 | 18.9 (14.8−23.0) | 36 | 10.3 (7.1−13.5) | 90 | 25.7 (21.1−30.3) |
| 9 | 418 | 54 | 12.9 (9.7−16.1) | 63 | 15.1 (11.6−18.5) | 192 | 45.9 (41.2−50.7) | 107 | 25.6 (21.4−29.8) | 40 | 9.6 (6.7−12.4) | 99 | 23.7 (19.6−27.8) |
| 10 | 445 | 72 | 16.2 (12.8−19.6) | 67 | 15.1 (11.7−18.4) | 217 | 48.8 (44.1−53.4) | 113 | 25.4 (21.3−29.4) | 62 | 13.9 (10.7−17.1) | 128 | 28.8 (24.6−33.0) |
| 11 | 515 | 94 | 18.3 (14.9−21.6) | 71 | 13.8 (10.8−16.8) | 238 | 46.2 (41.9−50.5) | 146 | 28.3 (24.5−32.2) | 87 | 16.9 (13.7−20.1) | 148 | 28.7 (24.8−32.6) |
| 12 | 561 | 118 | 21.0 (17.7−24.4) | 65 | 11.6 (8.9−14.2) | 311 | 55.4 (51.3−59.5) | 173 | 30.8 (27.0−34.7) | 124 | 22.1 (18.7−25.5) | 199 | 35.5 (31.5−39.4) |
| 13 | 577 | 127 | 22.0 (18.6−25.4) | 65 | 11.3 (8.7−13.8) | 309 | 53.6 (49.5−57.6) | 173 | 30.0 (26.2−33.7) | 138 | 23.9 (20.4−27.4) | 208 | 36.0 (32.1−40.0) |
| 14 | 343 | 69 | 20.1 (15.9−24.4) | 34 | 9.9 (6.8−13.1) | 181 | 52.8 (47.5−58.1) | 96 | 28.0 (23.2−32.7) | 123 | 35.9 (30.8−40.9) | 132 | 38.5 (33.3−43.6) |
| 0−14 | 4.378 | 682 | 15.6 (14.5−16.7) | 619 | 14.1 (13.1−15.2) | 1.929 | 44.1 (42.6−45.5) | 1.061 | 24.2 (23.0−25.5) | 668 | 15.3 (14.2−16.3) | 1.200 | 27.4 (33.3−43.6) |
The mean duration of symptoms in the overall sample was 4.4 days (SD, 3.1; median, 3 days); the mean duration of symptoms by age group can be found in Table 4. In 164 patients the symptoms lasted more than 14 days; Table 5 presents the odds ratios for a prolonged duration of symptoms, among which the odds ratio for the association with pulmonary disease was statistically significant.
Odds ratios for a duration of symptoms of 14 or more days based on variables under study.
| Odds Ratio | 95% CI* | |
|---|---|---|
| Sex | ||
| Male/female | 0.87 | 0.68−1.12 |
| Age | ||
| <1 | Reference | Reference |
| 2−3 | 0.87 | 0.50−1.53 |
| 4−6 | 0.25 | 0.14−0.46 |
| 7−10 | 0.48 | 0.28−0.80 |
| 11−14 | 1.05 | 0.65−1.70 |
| Presence of risk factors | ||
| Yes/No | 1.42 | 1.01−1.99 |
| Immunosuppression | ||
| Yes/No | Could not be assessed | |
| Heart disease | ||
| Yes/No | 1.80 | 0.56−5.80 |
| Pulmonary disease (including asthma) | ||
| Yes/No | 1.67 | 1.14−2.45 |
| Obesity | ||
| Yes/No | 1.45 | 0.86−2.48 |
As regards followup, 9837 patients (98.2%) had favourable outcomes at home. Eighteen patients were admitted to hospital (admission rate, 0.18%), their characteristics can be found in Table 6. The most frequent reason for admission was fever, and the odds ratio for admission was 2.80 in the subset of patients with fever (95% CI, 1.11–7.06). There was 1 case of MIS-C in a patient who was admitted to the inpatient ward (0.01%). None of the patients died.
Admitted patients.
| Patient | Unit of hospitalization | Age (years) | Sex | Reason for admission |
|---|---|---|---|---|
| 1 | Inpatient ward | <1 | Female | Fever |
| 2 | Inpatient ward | <1 | Female | Fever |
| 3 | Inpatient ward | <1 | Female | Fever |
| 4 | Inpatient ward | <1 | Male | Fever |
| 5 | Inpatient ward | <1 | Male | Fever |
| 6 | Inpatient ward | <1 | Male | Diarrhoea (observation in <1 month) |
| 7 | Inpatient ward | <1 | Male | Cold symptoms (observation in <1 month) |
| 8 | Inpatient ward | 2 | Male | Fever |
| 9 | Inpatient ward | 2 | Male | Laryngitis |
| 10 | Inpatient ward | 2 | Female | Pneumonia |
| 11 | Inpatient ward | 4 | Female | Vomiting and diarrhoea |
| 12 | Inpatient ward | 9 | Male | Observation (high-risk patient: cancer) |
| 13 | Inpatient ward | 10 | Female | Multisystemic inflammatory syndrome |
| 14 | Inpatient ward | 10 | Male | Bronchospasm |
| 15 | Inpatient ward | 10 | Female | Observation (high-risk patient: transplant recipient) |
| 16 | Inpatient ward | 13 | Female | Bronchospasm |
| 17 | Inpatient ward | 14 | Male | Admitted for other reason and PCR turned positive |
| 18 | Intensive care | 1 | Female | Bronchospasm |
Our study analysed the symptoms developed by children with SARS-CoV-2 infection in Spain during the second, third and early fourth waves of the pandemic, in the context of a developed country with a public primary care system that serves the majority of the paediatric population. The cases are likely to be representative of the clinical picture of the disease in the community, given that the study was conducted in the public health care system, which serves individuals of all socioeconomic levels throughout the entire national territory and there were no restrictions to the ordering of diagnostic tests during the period under study.
Our study, conducted in a very large sample in the community, confirms what has been described in previous publications4,5,16,17: in the paediatric population, COVID-19 is a mild and oligosymptomatic disease in the community. The salient findings were the frequent presentation with rhinorrhoea, which is not a symptom included in reference protocols18; its presence should be taken into account, despite its nonspecificity (frequent in infants, associated with allergic processes…), depending on the current epidemiological context. Another relevant finding was the low frequency of symptoms in children of intermediate ages, which could be attributed to the lesser ability to convey the symptoms they are experiencing and the pathogenic mechanisms of the disease in this age group,19,20 on which the evidence is growing. We also ought to highlight that from age 9 years, headache was the most frequent symptom (19.2% of cases), as previously described.21
One of the greatest challenges in fighting this disease is the presence of asymptomatic patients and their ability to spread the infection.22–24 The first studies published during the pandemic, when diagnostic tests were only available in the hospital setting, the proportion of asymptomatic patients in the paediatric population was reported to be as low as 14%15; since then, other studies using data obtained in the community and from population screening found percentages of 35%–50%13,17,25,26 and even as high as 71%–76.5% in the case of studies that only assessed symptoms at the time of diagnosis.27,28 In our study, in which a follow-up assessment of symptoms was conducted at 15 days, the percentage of asymptomatic patients was 43%.
As previously described,13,29 most children acquired the infection at home: in our sample, 70% of the cases. Consistently with previous studies, too,30–32 schools turned out to be safe settings, and we did not find significant differences in incidence between vacation periods and academic periods. The general consensus continues to be that the decision to close schools to control the COVID-19 pandemic should be a last recourse. The potential positive effects of this measure in the control of the pandemic do not appear to compensate the negative impact on physical health, mental health and academic performance in children,33 especially those who are most vulnerable.
All patients had favourable outcomes, and the incidence of complications was very low. The evidence to date has shown a higher rate of hospital admission in infants, especially in the presence of risk factors,34,35 which was also the case in our study.
The strengths of this study are that, unlike others, it analysed infections in the community, as opposed to in hospitalised or surgical patients, that it covered the full paediatric age spectrum, from 0 to 14 years, and that inclusion was independent of socioeconomic status, as primary care in Spain is universal and free. In addition, we analysed symptoms that were not included in many of the previous studies (gastrointestinal symptoms, asthenia, myalgia, etc.) and, since diagnostic tests were available in every case in which they were needed, we avoided the bias resulting from underdiagnosis that affected the evidence from the first wave of the pandemic, during which barely any cases were detected in children.
Among the limitations, we ought to mention the lack of a control group, as at the time of the study we did not consider its inclusion feasible due to the significant workload it would have entailed. There is also a risk of underdiagnosis due to several reasons: that tests may not have been ordered in some cases that collaborating paediatricians deemed mild or unlikely to be due to SARS-CoV-2, inadequate timing of the test (at the beginning or the end of the infection), or due to caregivers not seeking medical attention for children and/or not getting ordered tests done due to fear of restrictions to work or to home confinement. Lastly, confinement and social distancing measures and the use of masks implemented during the period under study may have modified the epidemiology of disease, which may preclude extrapolation of our results to situations in which these conditions are not present.
There is evidence that the measures taken to control the pandemic have caused some problems in the paediatric population: an increase in obesity,36 neurodevelopmental delay and mental health problems.33,37–40 The restrictions imposed on the paediatric population should be reconsidered now that we know that the course of COVID-19 in children is generally mild and given that, as of November 5, 2021, 88.7% of the target population has been vaccinated.
ConclusionCoronavirus disease 2019 is a mild disease in children and adolescents, with a high number of asymptomatic individuals in the community, and it has not been possible to identify a specific clinical picture that characterises this disease. The decision to close schools to control the pandemic should only be used as a last resort.
Conflicts of interestThe authors have no conflicts of interest to declare.
A los participantes de la Red de Investigación de Pediatría de Atención Primaria (PAPenRed): Abad Balaguer B, Acosta Navas B, Albañil Ballesteros MR, Alcaraz Melgarejo MD, Alcaraz Quiñonero M, Alcover Bloch E, Almazán Fernández de Bobadilla V, Álvarez García P, Álvarez Gómez J, Álvarez Mingorance P, Álvarez Vázquez E, Anfreu i Duat D, Angulo Moreno ME, Anllo Lago J, Aparicio Rodrigo M, Apolinar Valiente E, Ares Alvarez J, Arias López I, Arranz Sanjuan R, Arroyo Úbeda R, Asensi Monzo MT, Asensio Carretero S, Báñez Martín C, Barahona Robdon L, Barreiro Arceiz C, Barrios González EM, Bejarano López MA, Bercedo Sanz A, Bernabe Moyano MA, Bernad Albareda M, Bernad Usoz JV, Biosca Pàmies M, Bombín Granado JM, Bonet Garrosa A, Bravo Acuña J, Caballero Morales MA, Cabezas Tapia ME, Cadena Muñoz C, Callejas Pozo JE, Cantarero Vallejo MD, Capelo Miguez JM, Carmona Cedrés N, Caro Gómez A, Carrasco Sánchez P, Carrera Polanco M, Carvajal Urueña I, Casares Alonso I, Cascón Criado E, Castillo Marcalain A, Casuscelli LA, Chinarro Martínez P, Coello Torres Z, Comino Vázquez P, Company Maciá PM, Contreras Carreras D, Crespo Medina M, Cruz Navarro I, de Haro López MA, de la Serna Higuera PM, de las Heras Díaz-Varela C, del Castillo Aguas G, del Toro Calero C, Delgado Maireles M, Díaz Córcoles R, Díez Zaera O, Duelo Marcos M, Durán Iglesias C, Edo Jimeno MJ, Egea A, Escanciano Garcia Y, Escorial Briso-Montiano M, Escribano Romero MJ, Espinazo Ramos O, Espínola Docio B, Fernández Francés M, Fernández León A, Fernández López FJ, Fernández Pastor F, Fernández Tardáguila E, Fernández Villar AM, Fernández-Cuesta Valcarce MA, Galán Calvo MJ, Galardi Andonegui MS, Gallego Mingo N, Gallegos Miralles de Imperial T, Garach Gómez A, García Aparicio C, García Arroyo I, García Fraile ML, García García M, García Merino A, García Pérez R, García Rebollar CA, García Rodríguez AI, García Sánchez JA, García-Teresa García E, Garrido B, Gatell Carbo A, Gatius Tonda C, Gellioda Royo MJ, Gil Alexandres I, Gómez Casares R, Gómez Sorrigueta P, González Herrero M, González Martínez AJ, González Rodríguez MP, Gracia Alfonso MA, Grau García AI, Gros Esteban D, Gurbindo Arana C, Gutiérrez Abad C, Hernández Guillén R, Hernández Sáez MR, Hernando Helguero P, Hernando Zarate Z, Herranz Llorente M, Huguet Feixa A, Iglesias Griñant S, Jaramillo Hidalgo D, Javierre Miranda E, Jiménez Hereza JM, Juanes de Toledo B, Kirchschläger Nieto SB, Landín Iglesias G, Laso Alonso AE, Leal García MA, Ledesma Albarrán JM, Lobelle González C, Lobera Navaz MP, López Marcos M, López Méndez M, Lorente García-Mauriño A, Losa Frías V, Lozano Vergara M, Maestro Fernández R, Marco Puche A, Marfil Olink S, Margherita I, María Guerrero R, Martin Carballo G, Martin Ibáñez I, Martín Ramos S, Martínez Espligares L, Martínez Rubio MV, Martínez Ruiz MM, Meca Garrido JE, Medina Pomares J, Membrillo Lucena G, Méndez Bustelo MJ, Méndez Gallego S, Mendoza Álamo PI, Menéndez Bada T, Mengual Gil J, Merino Marcos MI, Mínguez Verdejo R, Monzón Bueno AI, Morell Bernabé JJ, Morellà Marroquin J, Moreno Conde AM, Moya Dionisio V, Muñoz Hiraldo ME, Muñoz Núñez MR, Murcia García J, Mustieles Moreno C, Navarro Cabañas G, Navarro Villalba EV, Navas Heredia CM, Niyubahwe I, Nocea Pulfer AM, Novo Rodríguez AI, Ordoñez Alonso MA, Ortells Ramón I, Padilla Esteban ML, Padilla Sánchez MC, Panizo Santos MB, Parejo Carranza R, Parejo Guisado S, Pavo Garcia MR, Pérez Candas JI, Poblet Puig M, Pozuelo Lima E, Prado Muñoz S, Prim Jaurrieta P, Puyuelo Del Val P, Quintana Fernández M, Quintanilla Sánchez M, Ramírez Parenteau S, Raventós Canet A, Regaña Velázquez M, Reinosa Díaz S, Rey del Castillo C, Reyes Medina M, Ripoll Lozano A, Ristol Perxés AM, Rodríguez Álvarez FJ, Rodríguez Baz MB, Rodríguez Contreras FJ, Rodríguez Fernández MM, Rodríguez Santana Y, Rodríguez-López Márquez A, Rodríguez-Salinas Pérez E, Romero García A, Ruano Fajardo CM, Ruiz Chércoles E, Ruíz Cuevas P, Ruiz J, Sáez de Lafuente Arriazu A, Saitua Benito A, Salvado Juncosa O, Sanantonio Valdearcos F, Sánchez Almeida E, Sánchez Andrés MT, Sánchez Cordero N, Sánchez Díaz MD, Sánchez Echenique M, Sánchez Moreno M, Sánchez Pina MC, Sánchez Precioso S, Sánchez-Prieto Emmanuel I, Sancho Madrid B, Santos García Cuellar MT, Satrústegui Gamboa F, Segura Ramírez DK, Sequera Bolivar MC, Sola Casado I, Suarez Fernández P, Suárez Vicent E, Suárez-Arrabal MC, Taboas Ledo MF, Téllez C, Tomás Aguirre B, Torres Álvarez de Arcaya ML, Torres Lloret C, Valencia T, Vaquerizo Pollino MJ, Vega Pérez MS, Vela Valldecabres C, Vera Domínguez MI, Vera Estrada M, Vigueras Abellán JJ, Vilas Rodríguez P, Vílchez Pérez JS, Villaizán Pérez C, Villanueva Farinós S, Viver Gómez S, Zardoya Santos P, Zugadi Zárate L.
Please cite this article as: Carballal-Mariño M, Balaguer-Martínez JV, García-Vera C, Morillo-Gutierrez B, Domínguez-Aurrecoechea B, Jimenez-Alés R, et al. Expresión clínica de la COVID-19 en pediatría de atención primaria: estudio COVIDPAP. An Pediatr (Barc). 2022;97:48–58.