Acute kidney injury (AKI) is a severe complication in paediatric intensive care units (PICUs) whose incidence varies (2%–10%) depending on the population under study and the diagnostic criteria applied, and is found more frequently in neonates.1 Different extracorporeal renal replacement therapies (RRTs) are available for its treatment, which are chosen based on the disease that led to AKI, the haemodynamic and respiratory status of the patient, and the resources available at the hospital.2 Peritoneal dialysis (PD) is the first choice in patients with coagulation disorders or in whom venous access is difficult, situations that are common in paediatric patients. However, despite being an inexpensive option that does not require staff specifically trained in haemodyalisis or much equipment, it is less effective than haemodialysis or other continuous RRTs in performing ultrafiltration (UF) and clearing solutes. In recent years, the technique of continuous flow peritoneal dialysis (CFPD) with a dual catheter system used in patients with chronic kidney injury in the 1960s and 70s has experienced a resurgence, with studies in the literature demonstrating higher clearances and UF compared to conventional PD, even in paediatric patients.3–6 Furthermore, the infusion of smaller volumes in the peritoneum results in improved haemodynamic and respiratory tolerability in critical patients.
The aim of this prospective, descriptive and observational study was to assess the feasibility, ultrafiltration efficacy and possible complications of dual-catheter CFPD in patients with AKI admitted to a PICU.
We included all patients admitted to a tertiary PICU between July 2013 and December 2014 that required PD for treatment of AKI after obtaining informed consent.
Description of the methodThe procedure was carried out under mild sedoanalgesia. Using the Seldinger technique, two percutaneous Cook® PD catheters were inserted, of size 8.5Fr in infants and 11Fr in one school-aged child. One of the catheters was inserted approximately midway between the umbilicus and the left anterior superior iliac spine, and the other midway between the umbilicus and the right anterior superior iliac spine, positioning its tip inferiorly to the other catheter to facilitate intraperitoneal drainage. We used abdominal ultrasonography in some patients with small volumes of ascitic fluid to determine the best site for insertion. The first catheter was used to infuse the dialysis solution BicaVera® 1.5% glucose at a rate of 10mL/kg/h with a continuous infusion pump, and the second catheter was connected to a urometer to measure the volume of dialysate drained via gravity on an hourly basis.
We collected and analysed data for the following clinical variables: age, sex, weight, underlying disease, Pediatric Risk of Mortality Score (PRISM), Kidney Disease Improving Global Outcomes Score (KDIGO), dialysate fill volume, glucose concentration of the dialysis solution, outflow volume, changes in fluid balance (FB) including diuresis and insensible losses, duration of treatment and associated complications.
We performed CFPD in five patients with clinical and laboratory signs of AKI in whom it was necessary to achieve a negative FB; four were in the post-operative period following surgery for congenital heart disease, and one had haemophagocytic syndrome secondary to infection by Epstein–Barr virus (EBV). Continuous flow peritoneal dialysis was chosen over other RRTs for the following reasons: its technical ease, as vascular cannulation is more difficult in young and unstable infants; its higher haemodynamic and respiratory tolerability, as lower fill volumes are required compared to intermittent dialysis techniques; and a lower risk of multiple transfusions and hypervolemia as well as of haemorrhage since heparin is not required, while it is in haemodialysis and other continuous RRTs.
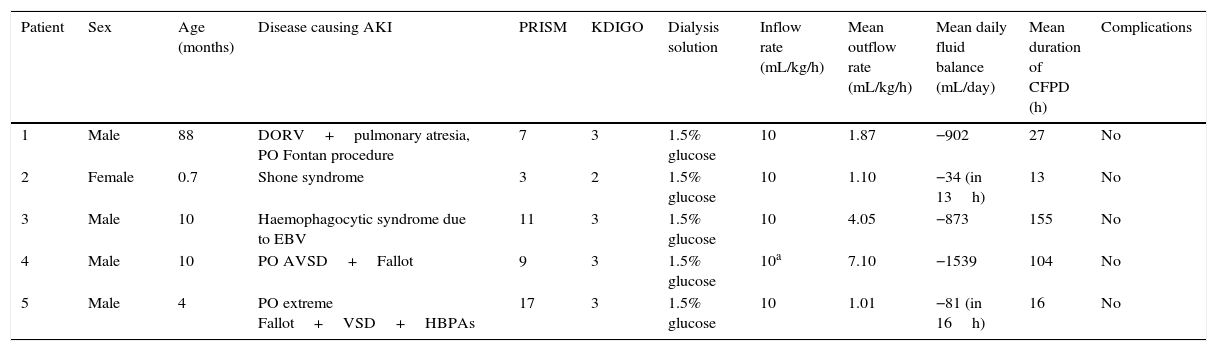
The ages of the patients ranged from 21 days to 7 years. Four of the patients were male. We achieved a mean dialysis outflow rate of 1.0–7.1mL/kg/h and a negative FB. We increased the dialysis inflow rate from 10 to 20mL/kg/h in one patient, and subsequently observed a higher outflow rate (mean rate, 9.9mL/kg/h). The criterion for discontinuation of CFPD was the improvement of spontaneous diuresis and renal function. Treatment duration ranged between 16 and 155h, with a median of 27h. We did not detect any complications associated with the technique, such as infection or catheter malfunction (Table 1).
Comparison of the series of clinical cases treated with a two-catheter continuous flow peritoneal dialysis technique.
| Patient | Sex | Age (months) | Disease causing AKI | PRISM | KDIGO | Dialysis solution | Inflow rate (mL/kg/h) | Mean outflow rate (mL/kg/h) | Mean daily fluid balance (mL/day) | Mean duration of CFPD (h) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 88 | DORV+pulmonary atresia, PO Fontan procedure | 7 | 3 | 1.5% glucose | 10 | 1.87 | −902 | 27 | No |
| 2 | Female | 0.7 | Shone syndrome | 3 | 2 | 1.5% glucose | 10 | 1.10 | −34 (in 13h) | 13 | No |
| 3 | Male | 10 | Haemophagocytic syndrome due to EBV | 11 | 3 | 1.5% glucose | 10 | 4.05 | −873 | 155 | No |
| 4 | Male | 10 | PO AVSD+Fallot | 9 | 3 | 1.5% glucose | 10a | 7.10 | −1539 | 104 | No |
| 5 | Male | 4 | PO extreme Fallot+VSD+HBPAs | 17 | 3 | 1.5% glucose | 10 | 1.01 | −81 (in 16h) | 16 | No |
AKI, acute kidney injury; AVSD, atrioventricular septal defect; VSD, ventricular septal defect; HBPAs, hypoplastic branch pulmonary arteries; PO, post operative; DORV, double outlet right ventricle; EBV, Epstein–Barr virus.
In this sample of patients, we found that CFPD with a dual catheter system for RRT was easy to implement, efficient, and safe due to the absence of associated complications.
Please cite this article as: Armero G, Benito S, Segura S, Jordan I, Cambra FJ. Diálisis peritoneal de flujo continuo en una unidad de cuidados intensivos pediátricos. An Pediatr (Barc). 2016;84:339–341.