Currently, kangaroo mother care (KMC) is an intervention whose implementation in clinical practice varies widely. The aim of this document is to gather the latest evidence-based recommendations in an attempt to reduce interprofessional variation and increase the quality of neonatal care.
MethodsThe document was developed following the guidelines provided in the Methodological Manual for the Development of Clinical Practice Guidelines of the National Health System: formulation and prioritization of clinical questions, literature search, critical reading, development of the document and external review. The target population was preterm (PT) and/or low birth weight (LBW) newborn infants admitted to a neonatal unit.
RecommendationsBased on the current evidence, recommendations have been issued to address 18 clinical questions regarding the impact of KMC (morbidity and mortality, physiological stability, neurodevelopment, feeding, pain, families), including infants with vascular access or respiratory support devices. It also describes the KMC procedure (transfer, positioning), the facilitators and barriers related to the implementation of KMC and how to implement KMC in extremely preterm newborns (less than 28 weeks of postmenstrual age in the first days of life).
ConclusionsKangaroo mother care is a beneficial practice for PT infants, LBW infants and their families. The implementation of these recommendations may be useful in everyday clinical practice and may improve KMC outcomes and the quality of care provided to neonatal patients.
Actualmente el método madre canguro (MMC) es una intervención con una alta variabilidad clínica en su aplicación. Este documento ha tenido como objetivo aunar las últimas recomendaciones basadas en la evidencia científica, para intentar disminuir la variabilidad interprofesional e incrementar la calidad de los cuidados al paciente neonatal.
MétodosSe han seguido las directrices descritas en el Manual Metodológico para la elaboración de Guías de Práctica Clínica del Sistema Nacional de Salud: redacción y priorización de preguntas clínicas, búsqueda bibliográfica, lectura crítica, elaboración del documento y revisión externa. La población a la que se dirige son los recién nacidos pretérmino (RNPT) y/o de bajo peso (RNBP) ingresados en una unidad neonatal.
RecomendacionesEn base a la evidencia existente, se proponen recomendaciones para 18 preguntas clínicas sobre el impacto del MMC (morbimortalidad, estabilidad fisiológica, dolor, neurodesarrollo, alimentación, dolor, familias), incluyendo a los portadores de dispositivos venosos o respiratorios. También recoge el procedimiento del MMC (transferencia, postura), los facilitadores y barreras para su implantación y su aplicación en prematuridad extrema (menores de 28 semanas de gestación en los primeros días de vida)
ConclusionesEl método madre canguro es una práctica beneficiosa para los RNPT, RNBP y sus familias. El uso de las recomendaciones aportadas podrá ayudar en la práctica clínica diaria y quizás se consiga mejorar los resultados del método madre canguro y la calidad de los cuidados prestados al paciente neonatal.
The kangaroo mother care (KMC) method is an effective and safe practice for all newborn (NB) infants during the stay in the neonatal unit.1 However, at present it is an underutilised approach in preterm (PT) infants, with substantial heterogeneity in its implementation in clinical practice, despite the existing evidence and proof of its beneficial effects. There is significant variation in the time it is initiated, the criteria to establish its indications, the duration and frequency of it or the required material resources.2 Although the World Health Organization (WHO) recommends initiation of KMC in PT and low birth weight infants (LBW) infants as soon as possible after birth and for as long as possible (between 8 and 24 h a day),1 guidelines are still needed to standardise this practice and improve the implementation, quality and safety of KMC nationwide in neonatal units in Spain. After consulting with experts, the working group that developed the document chose the term KMC to encompass the techniques referred to as kangaroo care, kangaroo method, kangaroo position, skin-to-skin contact or skin contact. The development of the document was an initiative promoted by the Group on Infant- and Family-Centred Developmental Care of the Sociedad Española de Enfermería Neonatal (Spanish Society of Neonatal Nursing, SEEN). This article is a summary of a more detailed consensus document that has a total of 95 pages.
MethodsDesignThe document was developed following the guidelines provided in the Methodological Manual for the Development of Clinical Practice Guidelines of the National Health System of Spain.3
Target populationAll preterm low-birth-weight infants admitted to a neonatal unit.
Development processThe group formulated and prioritised clinical questions applying the PICO model (population, intervention, comparison and outcomes). This was followed by a literature search (September 2022−23) in three databases—Cochrane Database of Systematic Reviews, Medline (through PubMed), CINAHL and Scopus—using Medical Subject Headings (MeSH) and natural language terms (Appendix A, Supplemental Material 1) combined with the Boolean operators AND, OR and NOT.
Subsequently, working groups were established for the peer review of the selected documents and retrieval of information. The quality of the evidence was assessed with the Critical Appraisal Skills Programme, Spanish version (CASPe) or, in the case of descriptive studies, the MinCir checklist. Each question was answered separately giving priority to the most recent and highest quality evidence. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework was used to assess the quality of the body of evidence, categorising it in 4 levels: high, moderate, low and very low, to then set the strength of recommendation for each question (strong/weak) (Table 1).
GRADE system for rating quality of evidence.
| Level of quality | Definition |
|---|---|
| High | Very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | Moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | The true effect may be substantially different from the estimate of the effect. |
| Very low | The true effect is likely to be substantially different from the estimate of effect. |
| Strength of recommendation | |
| Strong | Evidence that the benefits of the intervention clearly outweigh the harms or that the harms clearly outweigh the benefits. |
| Weak | Uncertainty about the benefits and harms or benefits and harms are equivalent, independently of the quality of the evidence. |
Lastly, the document underwent external review by other health care professionals in Spain and abroad as well as family organizations.
Working groupThe document was developed by the working group on Infant- and Family-Centred Developmental Care of the SEEN, composed of nurses with expertise in neonatal care and evidence-based nursing.
Clinical questionsImpact of kangaroo mother careWhat is the impact of KMC on mortality?Evidence
- •
Reduction in morbidity, mortality and nosocomial infection (high and strong).
Summary: it reduces morbidity and mortality, especially if initiated within 24 h of birth and performed for at least 8 h a day (RR, 0.68; 95% CI, 0.53−0.86),4 as it significantly reduces the incidence of severe infections (RR, 0.85; 95% CI, 0.79−0.92).4
What is the impact of KMC on physiological stability?
Evidence
- •
The KMC stabilizes the heart rate (HR), the respiratory rate and the oxygen saturation (SatO2) and therefore contributes to stabilizing and improving cardiovascular and respiratory function in PT infants (high and strong).
- •
KMC optimise cerebral oxygen saturation (low and strong).
Summary: reduction in apnoeic episodes (relative risk [RR], 0.41; 95% CI, 0.22−0.78),6 decrease in respiratory rate (by 3 bpm) (95% CI, −5.15 to −1.19), increase in SatO2 by 0.9% (95% CI, 0.35–1.45) and maintenance of temperature in normal range.5,7 Improvement in cerebral oxygen saturation (P < .01)8 and cerebral blood flow,9 which could contribute to reducing the risk of intraventricular haemorrhage (IVH), although there are no studies in the current literature that directly assessed the association of these 2 variables.
What is the impact of KMC on neurodevelopment?Evidence
- •
Improved sleep architecture (moderate and strong).
- •
Improved neurodevelopmental outcomes (high and strong).
Summary: During KMC, there is an increase in non-REM sleep (P < .001), with a reduction in wakings, indeterminate sleep and episodes of apnoea and hypoxaemia, with a more mature sleep pattern (P = .034).10 In the medium/long term, there is evidence of improved outcomes in cognitive function (P = .023), language skills (P = .014), academic performance, intellectual ability (P = .009) and family (P = .00014) and social (P < .001) adaptation, with a reduction in potential adverse neurodevelopmental outcomes (P = .004).11
What is the impact of KMC on nutrition?Evidence:
- •
It promotes breastfeeding initiation and maintenance and weight gain, and improves oral tolerance in infants receiving early enteral feeding (high and strong).
Summary: it is associated with an increased rate of exclusive breastfeeding at 6 months of corrected age (OR, 14.6; 95% CI, 3.5–60.9).12 Implementation of KMC for more than 3 h is associated with a reduction in the duration of parenteral nutrition (9 versus 12.5 days; P < .001) and in the prevalence of feeding intolerance (74% vs. 54%; OR, 0.42; 95% CI, 0.22−0.76).13 Implementation for more than 6 h is associated with increased weight gain (8.9 g/day; 95% CI, 8.14–9.84) and linear growth (0.29 cm/week; 95% CI, 0.15−0.43).14
What is the impact of KMC on the perception of pain?Evidence
- •
Implementation of KMC and KMC delivery by a parent is associated with a reduction in the pain response of PT infants (moderate and strong) without need of simultaneous administration of sucrose (high and strong).
Summary: KMC provides multisensory stimulation that inhibits nociceptive signals and promotes release of oxytocin, with an improvement in SatO2 (1.73; 95% CI, −0.53 to 3.99) and reductions in the HR (mean difference, −10.78; 95% CI, −13.63 to −7.93), duration of crying (by 34.16 s; 95% CI, −42.86 to −25.45) and score in the Premature Infant Pain Profile (PIPP) scale (P ≤ .01).15,16 It is effective for pain control, with statistically significant results (P = .002) and superior performance compared to sucrose. Containment and sucking at the breast are effective interventions on their own.
What is the impact of KMC on family health?Evidence
- •
It promotes attachment and bonding between the PT infant and the family (moderate and strong).
- •
Decreases maternal anxiety, stress and depression (high and strong).
Summary: early interaction with the PT infant improves attachment (P < .001) and reduces maternal stress (P < .001)17 and the risk of postpartum depression (RR, 0.76; 95% CI, 0.59−0.96).18
Candidates for kangaroo mother careIs KMC possible in PT infants with ventilatory support?Evidence
- •
There is no evidence of adverse events during KMC in PT infants receiving ventilatory support (invasive or non-invasive) (moderate and weak).
- •
KMC is not associated with an increase in unplanned extubation (moderate and strong).
Summary: The PT infants with ventilatory support (invasive or non-invasive) remain haemodynamically stable during KMC (target range: SatO2, 90%–95%; HR > 80 bpm).19 There have been no reports of increased frequency of unplanned extubation, neuroventilatory uncoupling or cardiopulmonary adverse events (bradycardia or desaturation episodes).20–22 Adequate training of health care staff and establishment of an infant transfer protocol are needed to prevent unplanned extubation.
Is KMC safe in infants with central venous lines?Evidence
- •
There is no evidence of adverse events associated with KMC in PT infants with central venous catheters (moderate and strong).
- •
KMC was not associated with the frequency of accidental catheter withdrawal (central or peripheral) or catheter-related bloodstream infection (moderate and strong).
Summary: The proportion of accidental withdrawal of the central venous catheter does not increase significantly during KMC or infant transfer,22 even in infants with umbilical catheters. There are also no differences in the incidence of complications (haemorrhage or migration) or the risk of bacterial colonization.23,24
Implementation of KMCWho can perform KMC?Evidence
- •
It is recommended that, whenever possible, the mother be the main provider of KMC, while also promoting the involvement of the father/partner in KMC (moderate and strong).
- •
Provision of KMC by another family member is recommended if the parents are not present or to offer parents a break (moderate and weak).
Summary: there are no differences in physiological, biochemical or behavioural outcomes in PT newborns based on whether KMC is delivered by the mother or father.25 Kangaroo mother care is more effective and quicker in relieving procedural pain when delivered by the mother as opposed to the father.25 There are no differences in HR, SatO2, respiratory rate or temperature when KMC is provided by the maternal grandmother vs. the mother(during KMC: HR 137.63 versus 139.12; respiratory rate 43.05 versus 44.25; SatO2 96.60 versus 96.47; PT infant temperature 37.05 versus 37.04).26
When and in what amount should KMC be delivered?Evidence
- •
Immediate initiation of KMC (within 1 h of birth) is recommended or, otherwise, as soon as possible (high and strong).
- •
A minimum of 6−8 hours of KMC a day is recommended (moderate and strong).
Summary: Early initiation of KMC (before 24 h post birth) compared to late initiation has been found to reduce mortality at 28 days post birth (RR, 0.78; 95% CI, 0.66−0.92) and is associated with a higher prevalence of exclusive breastfeeding at discharge (RR, 1.12; 95% CI, 1.07–1.16).4
What resources are required?Evidence
- •
The use of ergonomic support (wrap) has been suggested because it increases the time spent delivering KMC and the comfort and satisfaction of the parent and PT infant (moderate and weak).
- •
The use of an infant cap is suggested, although it depends on the postmenstrual age and previous temperature. A blanket or cover can also be used, covering the infant’s head (low and weak).
Summary: We did not find evidence assessing the use of a tubular abdominal support stretch band. Two KMC ergonomic support products that can be customised have been evaluated (CarePlus Wrap and Sarbebe), evincing a 20% increase in the time spent in KMC (P = .03) and increased maternal satisfaction (4.9 [0.3] versus 4.11; P < .001).27,28 When it comes to the use of a cap, a study conducted in Africa in 300 PT infants with birth weights of less than 2500 g during KMC in the 7 days of life found a mean time spent in the normal temperature range of 55% in the group wearing a cap (SD, 24) compared to 56% in the group without a cap (SD, 24) (OR, 0.95; 95% CI, 0.86–1.04), and there were also no differences in the frequency of hypothermia or hyperthermia episodes.29
What position should be used for KMC?Evidence
- •
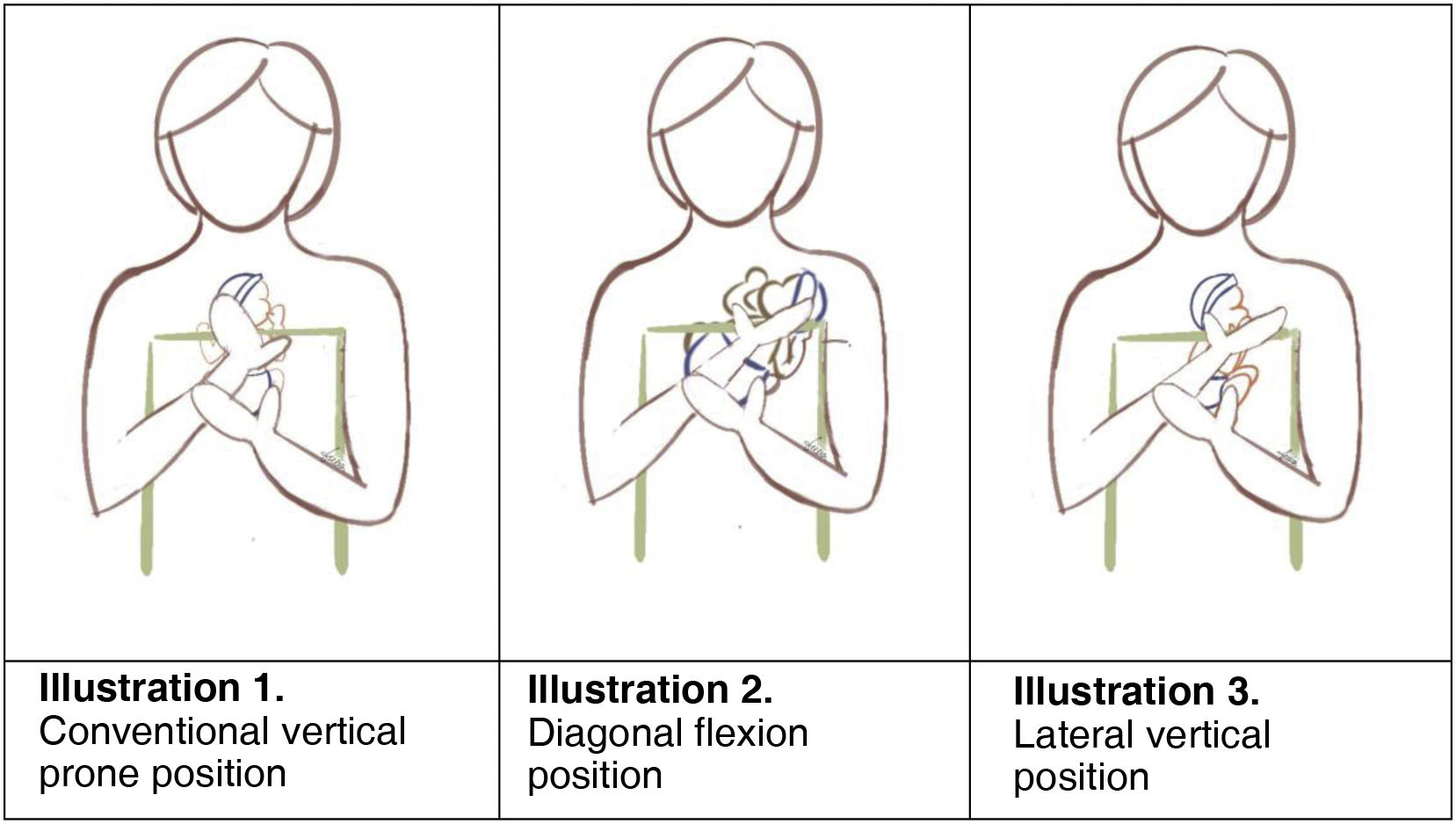
The use of positions alternative to the vertical prone position has been suggested (Fig. 1, illustration 1): diagonal flexion position (may promote mother-infant communication and interaction and longer time in deep sleep) (Fig. 1, illustration 2) and lateral vertical position (to keep extremely PT infants in the normal temperature range with stable HR and SatO2) (Fig. 1, illustration 3) (moderate and weak).
- •
Avoiding the vertical prone position in PT newborns and using the lateral vertical position for KMC in the first 72 h as an alternative has been suggested (moderate and weak).
- •
Using a mirror or the front camera of a mobile phone or tablet is suggested to be able to see the face of the PT infant (expert opinion and strong).
Summary: the general recommendation is vertical positioning of the infant between the breasts/at middle of chest, witih the head upright and turned to one side, the hips flexed and abducted (frog position) and the arms flexed.30 However, studies that have assessed supported diagonal flexion positioning for KMC compared to conventional vertical positioning have found an increase in the time in deep sleep with the diagonal position vs. the vertical position (22% vs. 6%) and a decrease in the time spent in the drowsiness state (58% vs. 70%). The HR and respiratory rate decreased in both groups, but the mean SatO2 was 96 in the diagonal position (1.9) versus 98.4% (1.3) in the vertical position (P = .018). The score in the Edinburgh Postnatal Depression Scale (EPDS) was also lower in the diagonal versus the vertical group (7 [4.4] vs. 9 [2.7]; P = .115).31 There is no solid evidence that the supine lateral head position increases the risk of IVH,32 but there is evidence that keeping the head in the midline position can improve cerebral oxygen saturation and blood flow.8,9
How should the baby be transferred?Evidence
- •
It is recommended that 2 individuals (at least one a health care provider) collaborate in transferring the infant, although it depends on the health condition and age of the baby, attached devices, intubation status and parental autonomy/skills (low and weak).
- •
The following are recommended: assessment of autonomy of parents and stability of PT infants, and standardization of the technique to establish whether the infant will be transferred while the KMC provider is standing or sitting (low and weak).
- •
In the seated position, when the baby is transferred from the incubator to the KMC provider, keeping the infant in the infant positioner during the transfer is recommended (low and weak).
- •
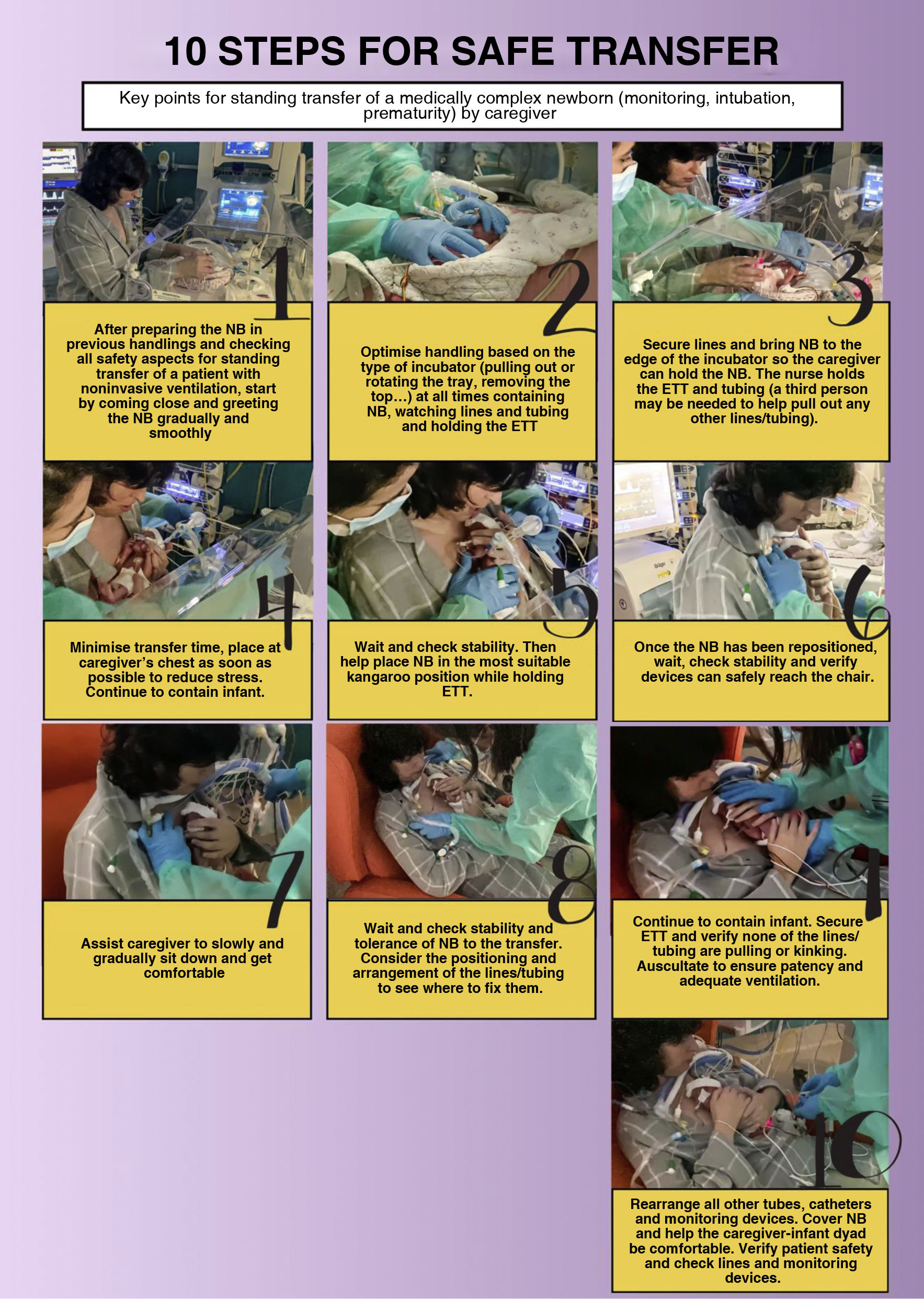
In the case of intubated infants, it is recommended that at least 2 individuals assist in the transfer (one handling the endotracheal tube and the other the circuit tubing) without disconnecting the patient. At the chair, the circuit tubing should be fixed above the shoulder of the KMC, making it just loose enough to allow the PT infant to move. It should be fixed at an easily accessible spot with a method allowing quick removal in the event of an emergency (low and weak) (Fig. 2, Illustration 2).
Summary: at least one nurse and one other health care professional are required to move the infant, but there is no consensus on which type of transfer, standing or sitting, is safer.33 Standing transfer is less stressful because it takes less time and produces less physiologic disorganization and thermal and behavioural distress. Another study found a smaller decrease in the SatO2 during transfer by standing parents (2.9%) compared to seated parents (2%), although recovery of baseline values with the parents is immediate. When parents conduct the transfer, there is a smaller increase in HR compared to transfers conducted by staff, of 2% vs. 5% of the baseline.33 Other observational studies support the recommendation of transfer in the sitting position as more effective and less stressful (without contributing specific data), insisting on establishing a standardised protocol to reduce the instability generated by transfers. When it comes to the use of positioners, there was no evidence in the consulted literature, which only included protocols and descriptions of their use in PT infants with ventilatory support.
Facilitators and barriers in KMCDoes having a guideline or protocol facilitate KMC?Evidence
- •
Establishing an institutional guideline or protocol is recommended to improve the implementation and application of KMC (moderate and strong).
Summary: having a protocol in place improves acceptance and increases the comfort of nurses in regard to KMC. On the other hand, its absence poses a barrier for KMC implementation, giving rise to worry and insecurity among health care professionals.34
Do facility design and severity level affect KMC?Evidence
- •
Implementation of KMC in private areas or rooms that can accommodate both parents and free access to the unit around the clock are recommended (high and strong).
Summary: The lack of adequate space and resources is a barrier that can interfere with the motivation and wellbeing of the parents and with the ability of professionals to implement KMC.34 To achieve optimal KMC, families should be allowed access to the infant 24 h a day.35 Family rooms appear to decrease maternal stress and facilitate maternal involvement, with mothers spending more hours a day with their infants.
Does specific training and clinical experience in neonatal nurses facilitate KMC?Evidence
- •
Training and clinical experience in KMC of neonatal nurses facilitates the implementation of KMC (moderate and strong).
- •
A positive perception of the competence of health care staff increases the willingness of parents to engage KMC (high and strong).
Summary: centres that provide specific training on KMC have a higher success rate in its implementation.36 Nurses who are more experienced in KMC support it more and are more successful at its implementation. The presence of specialised nurses decreases parental apprehension and facilitates parental involvement in the care of PT infants, thus facilitating the implementation of KMC. Easy access to health care staff and parental education on KMC are facilitators, and lack of support with KMC implementation, lack of education on KMC or merely telling parents to implement it are barriers to caregivers accepting KMC.
Does the perceived level of severity affect KMC?Evidence
- •
The level of severity perceived by nurses in the infant affects the implementation of KMC, especially if the nurse-to-patient ratio is low (high and strong).
- •
The severity perceived by parents has an impact on the frequency with which they provide KMC (high and strong).
Summary: nurses may be concerned about implementing KMC in clinically unstable infants, especially if the workloads are high and there is a lack of organizational support or clear guidelines.36 Families frequently experience anxiety and fear of hurting the infant in relation to the implementation of KMC.36 Maternal concern that the PT infant is currently or could become unstable may also pose a barrier to KMC.36,37
Extremely preterm infants and KMC
¿Is KMC safe in extremely PT newborns?Evidence
- •
Implementation of KMC as soon as possible is recommended if the condition of the infant allows it (low and strong).
- •
Use of a polyethylene bag is recommended prevent hypothermia during KMC in the first week of life, establishing need on a case-by-case basis (low and strong).
Summary: Few studies have assessed the efficacy and safety of KMC in infants born preterm before 28 weeks’ gestation. Some studies demonstrate maintenance of normal temperature (improvement of 0.2−0.3 °C compared to conventional care in incubator), haemodynamic stability and safety during KMC in extremely PT newborns.38,39 Use of a polyethylene bag during KMC also achieves maintenance of normal body temperature at 60 min of KMC.40
ConclusionThe kangaroo mother care approach is beneficial for PT and very LBW infants and their families, so it is important to promote its implementation in clinical practice. This consensus document, based on the current scientific evidence and the GRADE system, addresses the 18 clinical questions formulated about KMC regarding its impact, indication/eligibility, implementation, facilitators and barriers and its use in extremely preterm infants. The resulting set of recommendations may guide decision-making for health care professionals involved in neonatal care, thus reducing heterogeneity in care delivery and improving the quality and safety of KMC.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.