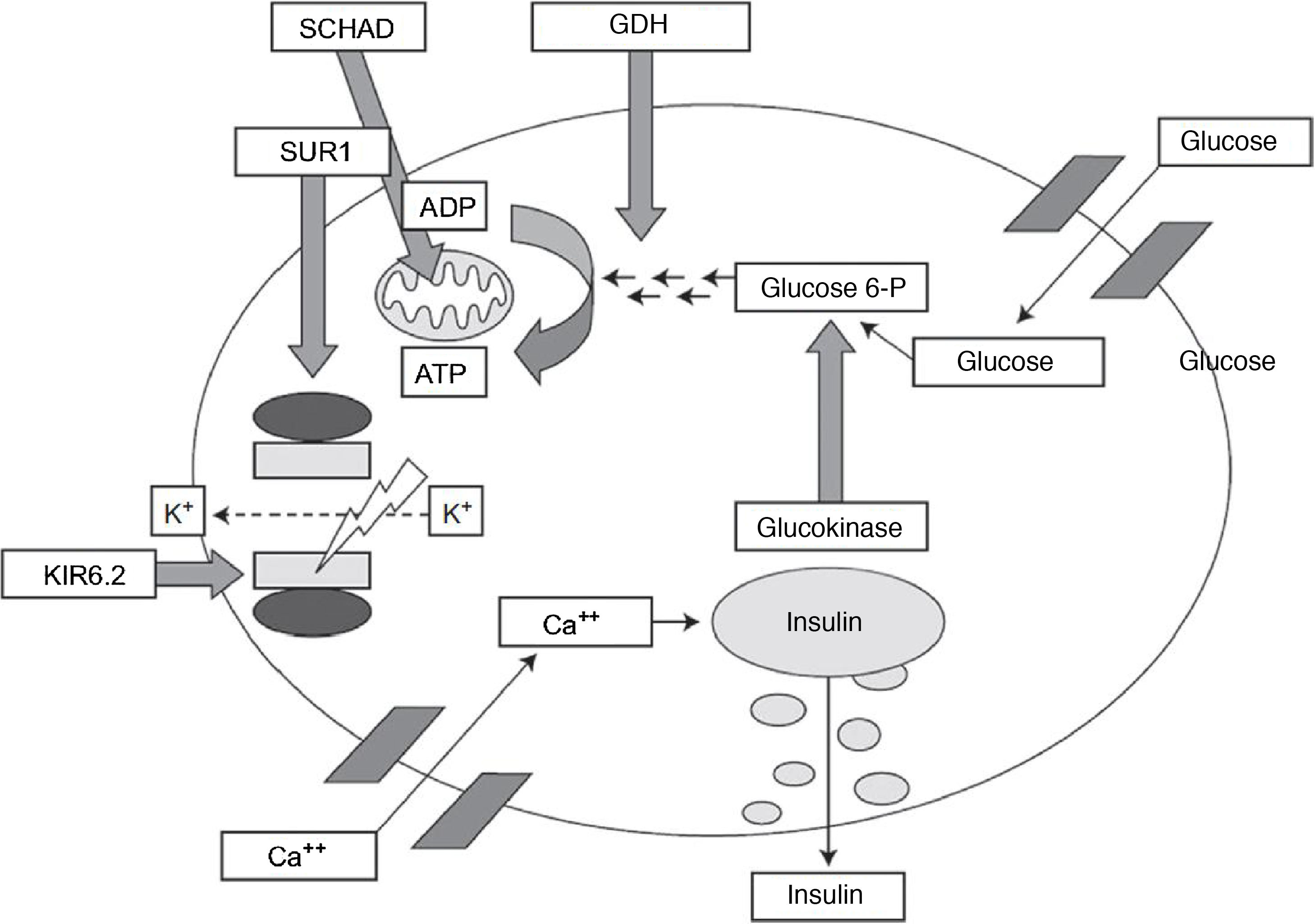
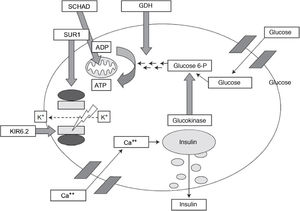
Congenital hyperinsulinism is the most frequent cause of persistent hypoglycaemia in the paediatric population. Early detection and treatment are essential to prevent the neurologic impairment associated with recurrent or prolonged episodes of hypoglycaemia.
Different genetic variants have been identified that cause this disorder, chiefly channelopathies and congenital metabolic disorders.1–3 The GLUD1 gene encodes the enzyme glutamate dehydrogenase 1, which delivers a signal involved in stimulating insulin secretion (Fig. 1). Five percent of cases of hyperinsulinism are caused by abnormalities in this gene.1
Pathophysiology of insulin secretion pancreatic beta cells.
Source: J. Guerrero-Fernández et al. Hiperinsulinismo congénito. Revisión de 22 casos.7
ADP, adenosine diphosphate; ATP, adenosine triphosphate; GDH, dehydrogenase Glucose 6 P, glucose 6-phosphate; KIR6.2, potassium channel subunit; SCHAD, short-chain-3-hydroxyacyl-CoA dehydrogenase; SUR1, sulfonylurea receptor.
Unlike the typical isolated episodes of hypoglycaemia, which tend to occur after prolonged periods of fasting or intense physical activity, hypoglycaemic episodes in these patients can also develop after meals, especially after consumption of protein. This disorder was traditionally known as hyperinsulinism-hyperammonaemia syndrome on account of its association with high serum levels of ammonia, but this feature is not present in every affected patient. The diagnosis is based upon a blood specimen obtained at the time of spontaneous or provoked hypoglycaemia, and the glycaemic response to glucagon.1,2
Treatment consists of dietary measures and pharmacotherapy, and the first-line drug is diazoxide.4,5
We present 1 case of congenital hyperinsulinism associated with a likely pathogenic variant of the GLUD1 gene that was also detected in 2 symptomatic relatives. The clinical expression of this variant in different members of the family will help elucidate the phenotype of this mutation.
The patient was a girl aged 17 months brought to the neurology clinic for evaluation of asthenia, hypersomnia and episodes of hypotonia over a 2-month period, especially following the night-time fast and not associated with a specific type of intake. The father, aged 40 years, had received a diagnosis of narcolepsy in childhood. We ordered blood tests, including determination of the level of ammonia, which was normal. The patient exhibited normal psychomotor development and an electroencephalogram revealed intercritical multifocal paroxysmal activity of moderate intensity with little diffusion.
One month later, the patient presented with decreased level of consciousness. The physical examination revealed a weight of 11.4kg (z=0.45), a height of 82cm (z=0.8), body mass index of 16.9 (z=−0.04) and was normal overall, except for a Glasgow coma scale score of 14/15. The blood test panel revealed serum levels of glucose of 27mg/dL, insulin of 14.7μIU/mL, ammonia of 28μmol/L and C-peptide of 3.7ng/mL, and the results of the ketone urine and blood tests were negative. The patient required administration of glucose at a dose of 11.9mg/kg/minute. A glucagon test was performed, and the positive results confirmed the suspicion of hyperinsulinism. Treatment with diazoxide was initiated at a dose of 8.6mg/kg/day and achieved a favourable response.
Genetic testing confirmed the presence of a variant of the GLUD1 gene, the heterozygous mutation c.1466C>A in exon 11 that resulted in an amino acid change [p.(Pro489His)]. The same variant was detected in the father and a sister aged 4 years that had symptoms compatible with hypoglycaemia before meals. The results of genetic testing were normal in the mother. The father also reported episodes of asthenia and sweating after protein consumption.
Congenital hyperinsulinism manifests with inappropriate insulin secretion that can cause severe episodes of hypoglycaemia. It has a genetic basis, but the pathogenic change is only identified in 50% of cases.4,6 The variants detected most frequently are those associated with dysfunction of KATP channels, followed by changes in the GLUD1 gene. The family described in this article had a change in the GLUD1 gene, with the particularity that this was a novel variant of unknown clinical significance. The c.1466C>A variant and the resulting amino acid substitution p.(Pro489His) are not described in the dbSNP or ClinVar databases, although analyses to predict its functional impact suggested it was likely pathogenic. The phenotypic expression in the family we describe here confirms the clinical significance of this variant at different stages in life. In fact, the father of the index case had symptoms in early childhood, but was given a diagnosis of narcolepsy. The diagnosis we made at a later date suggests that what he was experiencing at the time were most likely symptomatic episodes of hypoglycaemia.
The severity of this disease is usually associated with the causative variant, and therefore genetic testing is essential to establish the prognosis and develop a treatment plan based on the identified abnormality. The description of the clinical features found in patients with novel variants of the genes involved in congenital hyperinsulinism could help establish a comprehensive picture of the genotype-phenotype association. This is particularly true of descriptions of multiple cases in a single family, like the one presented in this article.
Fortunately, this type of genetic changes respond to treatment, as was the case in our patient. On the other hand, changes in the ABCC8 gene usually cause forms of hyperinsulinism that are refractory to diazoxide.1,5 In such cases, treatment with octreotide is indicated. Children that do not respond to either treatment may require intensive care with continuous tube feeding or surgery.5
In conclusion, the identification of the causative variant in patients with congenital hyperinsulinism is crucial to the interpretation of the clinical phenotype and the selection of the most appropriate treatment. The c.1466C>A variant of the GLUD1 gene described here is associated with a classic hyperinsulinism phenotype.
Please cite this article as: Herrera Azabache K, Muñoz Bermúdez Z, Ferrández Mengual D, Nso-Roca AP. Hiperinsulinismo congénito en tres pacientes de la misma familia. Ampliando el genotipo de esta enfermedad. An Pediatr (Barc). 2021;95:123–124.
Previous presentation: This case was presented at the XXXV Congress of the Sociedad Valenciana de Pediatría; April 6, 2019; Calpe, Spain.