Respiratory syncytial virus (RSV) is the most frequent causative agent of lower respiratory tract infections in children. In some cases, it can cause severe cardiovascular or central nervous system disease, such as myocarditis or necrotising encephalitis.1 We present the case of a girl with acute myocarditis and complete heart block (CHB) associated with RSV infection.
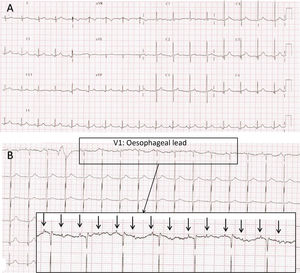
The patient was a 1-month old female infant admitted to the paediatric intensive care unit (PICU) with severe bronchiolitis secondary to RSV infection and receiving noninvasive mechanical ventilation. A few hours after admission, the patient had an episode of prolonged bradycardia requiring intubation. After intubation bradycardia persisted (60–70 bpm) and the patient eventually developmend arterial hypotension. Infusion of adrenaline was initiated followed by performance of an electrocardiogram (ECG), which revealed a narrow complex bradycardia (Fig. 1). Each QRS complex was preceded by a P wave, but there seemed to be blocked P waves hidden in the T wave. An oesophagus ECG was performed that confirmed the presence of CHB (Fig. 1B). Intravenous infusion of isoproterenol was initiated at a dose of 0.2 μg/kg/min, achieving an increase of the heart rate (HR) to 120–130 bpm and resolution of hypotension. Twenty-four hours later, the patient developed ventricular dysfunction with a left ventricular ejection fraction (LVEF) of 42%, lactate elevation (3.8 mmol/L), pulmonary oedema and elevation of troponins (peak ultrasensitive cardiac troponin T, 27 ng/L), compatible with myocarditis. The patient required milrinone (0.5 μg/kg/min) and adrenaline (up to 0.3 μg/kg/min). A workup oriented to myocarditis was including a panel to identify anti-Ro and anti-La antibodies in the infant and the mother, serological tests for Lyme disease, blood, urine and cerebrospinal fluid (CSF) cultures, PCR tests for detection of viral and bacterial pathogens in blood, stool and nasopharyngeal aspirates, tests of serum amino acids and acylcarnitine profile analysis. The patient was treated with intravenous immunoglobulin (1 g/kg for 2 days), carnitine, ribavirin and palivizumab. She exhibited a quick haemodynamic recovery, allowing discontinuation of adrenaline and milrinone on days 3 and 5, respectively. However, the CHB persisted. The patient received 2 separate courses of intravenous methylprednisolone (2 mg/kg for 5 days), without response. The patient could tolerate a HR of 60–75 bpm, so she did not need placement of a pacemaker. The PCR test for detection of RSV-B was positive for the nasopharyngeal aspirate sample and the blood sample collected during the acute phase. The patient has remained asymptomatic during the follow-up. The ECG at 3 months showed recovery of atrioventricular (AV) conduction, with evidence of a residual first-degree AV block, which remained 9 months after the initial event.
(A) Electrocardiogram recorded in the PICU at 24 h from admission. Narrow complex bradycardia with suspected high-grade atrioventricular block with 2:1 conduction. Blocked P waves “buried” in the T wave of the preceding heartbeat are difficult to discern.
(B) Electrocardiogram with oesophageal probe (lead V1) recorded immediately following the one shown in Fig. 1A. Features consistent with high-grade 2:1 atrioventricular (AV) block. Atria (arrows) and ventricular activity seem dissociated, as the AV interval changes from beat to beat.
Note: Since neonatal oesophageal electrodes were not available, we fitted an oesophageal lead as follows: we inserted a gastric tube previously rinsed with physiological saline, which served as the conductor, and without entirely removing the guide wire we connected it to the ECG lead and to the monitor. We slowly pulled the tube up towards the oesophagus while watching the ECG tracings to the point that atrial activity was detectable with the highest possible amplitude. The oesophageal tracings allow observation of amplified atrial activity and to analyse it independently from the QRS complex. This is very useful in the diagnosis of certain forms of supraventricular tachycardia or in case of conduction disorders when the AV conduction ratio is not clear in the surface ECG.
CHB is an unusual manifestation in the course of acute myocarditis. A recent study in 31 760 adults found an incidence of 1.1%, and its presence was associated with longer lengths of stay and a higher mortality.2 In 2014, Anderson et al3 analysed the impact of arrhythmia in the prognosis of paediatric myocarditis. Of the 2041 patients, 1.1% developed CHB, but this was not associated with a less favourable prognosis. It seems that CHB is more frequent in fulminant cases of acute myocarditis, with a prevalence that ranges between 14% and 71%.4,5 The CHB usually resolves during the acute phase of myocarditis, and most authors concur that CHB does not negatively impact the final outcome if it is treated correctly.
Cardiovascular disorders are the most frequent extrapulmonary manifestation of RSV infection. Subclinical myocardial damage, evidenced by troponin elevation, is frequent in severe cases of bronchiolitis. However, RSV is an infrequent cause of clinical myocarditis, accounting for approximately 1.8% of total cases.3 It appears that cardiac involvement in RSV infection tends to affect the conduction system. A review of 40 cases of CHB associated with myocarditis identified RSV in 2 of the 12 cases with a confirmed causative agent (16%), a proportion that was larger than expected.6 Esposito et al7 found evidence of conduction abnormalities in the Holter monitoring recordings of patients with mild to moderate bronchiolitis caused by RSV in the absence of signs of myocarditis. To our knowledge, only 6 cases of CHB associated with RSV have been reported in the previous literature (Table 1).8–12 Most were in male patients, and the CHB was permanent in 4 cases. Whether CHB is caused by direct injury or immune-mediated damage to the conduction system or secondary to myocardial inflammation has yet to be established. In the case presented here, CBH was the most important feature in the course of disease, developing rapidly during the infection by RSV and preceding the identification of cardiac dysfunction. In addition, CHB persisted for several months past the resolution of myocarditis.
Reported cases of complete heart block associated with infection by RSV.
| Author | Year | Age | Sex | Clinical myocarditis | Pacemaker | Resolution of block | Follow-up |
|---|---|---|---|---|---|---|---|
| Bairan8 | 1974 | 3 years | Male | Yes | Permanent | No | 1.5 years |
| Giles9 | 1976 | 15 years | Male | Yes | Temporary | Yes, in 20 days | 5 months |
| Menahem10 | 1985 | 3 years | Male | Yes | No | No | 4 years |
| Menahem11 | 2010 | 3 years | Male | No | Permanent | No | – |
| Karatza12 | 2017 | 10 months | Male | Noa | No | No | 5 years |
| Oulego-Erroz (presented case) | 2019 | 1 months | Female | Yes | No | Yes, in 3 months (residual 1st degree AVB) | 9 months |
In conclusion, paediatricians must be aware that RSV may affect the cardiac conduction system. Bradycardia of unusual duration or unknown origin should alert the clinician of the possibility of a high-grade atrioventricular block, with or without myocarditis. Early diagnosis and treatment are essential to prevent complications and improve outcomes. Although CHB associated with infection may be transient, in some cases it can progress and become permanent, and therefore requires close follow-up.
Please cite this article as: Oulego-Erroz I, de Castro-Vecino P, Ocaña-Alcober C, Gutiérrez-Marqués S, Martínez-Badás JP, Centeno-Jiménez M. Bloqueo auriculoventricular completo asociado a infección por virus respiratorio sincitial: presentación de un caso y revisión de la literatura. An Pediatr (Barc). 2021;94:417–419.