At this time there are still major questions about the characteristics of disease caused by the new coronavirus (COVID-19) in children as well as factors associated with the development of severe forms of the disease.
Study designRetrospective study including patients under 18 years of age admitted with SARS-CoV-2 infection from March 1 to April 30, 2020. Infection was confirmed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) or antibody testing. We describe the epidemiological and clinical data, laboratory and imaging findings, as well as treatment and outcome in these patients. In light of these findings, patients were classified into two severity groups and then compared.
ResultsThirty-nine children were included, with a median age of 9 years (range 12 days–16 years); 23 were boys. Cases with uncomplicated disease course (24) mostly presented to the emergency department (ED) with fever and/or respiratory symptoms without significant alterations in laboratory findings. Of the 15 children with a complicated course, 12 developed shock. In addition to fever, they frequently presented altered appearance, extreme tachycardia, abdominal pain, vomiting, diarrhea, rash, and/or conjunctival hyperemia. They also showed greater lymphopenia (p=0.001), elevated neutrophil/lymphocyte ratio (p=0.001), C-reactive protein (p<0.001), procalcitonin (p=0.001), D-dimer (p<0.001), and ferritin (p<0.001).
ConclusionsSARS-CoV-2 infection in admitted children presents with great clinical variability. When provided supportive care, patients with predominant respiratory symptoms without altered laboratory-test results generally have an uncomplicated course. Patients with complicated disease present mainly with fever and abdominal and/or mucocutaneous symptoms. Most develop shock. Elevation of inflammatory markers may allow for early detection and the final outcome is good.
En este momento existen todavía grandes interrogantes acerca de las características de enfermedad causada por el nuevo coronavirus (COVID-19) en los niños, así como acerca de los factores asociados al desarrollo de formas graves de la enfermedad.
MétodosEstudio retrospectivo que incluye pacientes menores de 18 años ingresados debido a infección por SARS-CoV-2. La infección fue confirmada por la reacción en cadena de la transcriptasa inversa-polimerasa (RT-PCR) en tiempo real o por serología. Describimos los datos epidemiológicos y clínicos, los hallazgos de laboratorio y de imágenes, así como el tratamiento y la evolución de estos pacientes. Los pacientes se clasificaron en dos grupos de gravedad y luego se compararon.
ResultadosSe incluyeron 39 niños, con una mediana de edad de nueve años (rango 12 días-16 años); 23 eran varones. Los casos con evolución no complicada (24) se presentaron en su mayoría con fiebre y/o síntomas respiratorios sin alteraciones significativas en los hallazgos de laboratorio. De los 15 niños con enfermedad complicada, 12 desarrollaron shock. Además de la fiebre, frecuentemente presentaban alteraciones de la apariencia, taquicardia extrema, dolor abdominal, vómitos, diarrea, erupción cutánea y/o hiperemia conjuntival. También mostraron mayor linfopenia (p=0,001), elevación de la proporción neutrófilos/linfocitos (p=0,001), proteína C reactiva (p<0,001), procalcitonina (p=0,001), dímero D (p<0,001) y ferritina (p<0,001).
ConclusionesLa infección por SARS-CoV-2 en niños ingresados se presenta con una gran variabilidad clínica. Cuando se les proporciona tratamiento de soporte, los pacientes con síntomas respiratorios que no tienen alteración de las pruebas de laboratorio, generalmente tienen una enfermedad no complicada. Los pacientes con enfermedad complicada se presentan principalmente con fiebre y síntomas abdominales y/o mucocutáneos, la mayoría desarrollan un shock. La elevación de los marcadores inflamatorios puede permitir una detección temprana y el pronóstico final es bueno.
In Spain, the first case of coronavirus disease 2019 (COVID-19), caused by the novel coronavirus known as SARS-CoV-2,1 was detected on January 31, 2020. In the weeks that followed, SARS-CoV-2 spread through the entire country, and the Community of Madrid was the region hit the hardest.2
In order to optimise health care resources, starting in March 20, 2020 urgent paediatric care was centralised at 2 tertiary care hospitals in this autonomous community. These 2 hospitals were tasked with providing emergency, inpatient and intensive care services to a population of approximately 1345000 individuals aged less than 18 years.
Although SARS-CoV-2 can infect individuals of any age, the overall incidence in the paediatric population has been estimated at 1–2%.3–5 The prevalence in Spanish children and adolescents found in a study conducted in May 2020 ranged from 1% to 5%.6 Children develop milder forms of disease and have better outcomes compared to adults.7
The symptoms described most frequently in children with infection by SARS-CoV-2 are similar to the symptoms of an upper respiratory tract infection,8 that is, cough, fever, sore throat, nasal discharge and myalgia.9 Gastrointestinal symptoms are also frequent in this population, including decreased appetite, nausea, vomiting and diarrhoea. A multisystem inflammatory syndrome has been described in children (MIS-C) with infection by SARS-CoV-2 that manifests with shock and may result in multiple organ failure secondary and is considered one of the most severe forms of COVID-19 in the paediatric population.10–12 This syndrome usually presents with high fever and gastrointestinal symptoms (severe abdominal pain is present in nearly all cases).
At present, the spectrum of diseased caused by infection by SARS-CoV-2 is not well defined in the paediatric population. There is a dearth of data on the course of disease and prognostic factors in hospitalised children. The aim of our study was to describe the epidemiological characteristics, different clinical presentations and risk factors for complicated disease in hospitalised children with COVID-19.
Patients and methodsSampleWe conducted a retrospective observational study at a tertiary care children's hospital, the Hospital Infantil Universitario Niño Jesús in Madrid, Spain, between March, 1 and April 30, 2020. The study was approved by the ethics committee of the hospital. All the data were collected with the informed consent of the parents or caregivers.
The inclusion criteria were:
- -
Patients aged less than 18 years that required admission due to infection by SARS-CoV-2.
- -
Infection by SARS-CoV-2 confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) in a nasal or pharyngeal swab sample or by detection of IgG antibodies in a blood sample.
The exclusion criteria were:
- -
Pre-existing oncological disease.
- -
Inpatient with a positive PCR test for detection of SARS-CoV-2, but who had not been admitted for management of this infection.
The criteria to define confirmed infection by SARS-CoV-2 in suspected cases have changed during the pandemic. We did not assess for potential infection in patients that did not meet the inclusion criteria. Before March 11, an RT-PCR test was performed in hospitalised patients if they exhibited symptoms of an acute respiratory tract infection, had travelled to areas with known community spread or had had close contact with a probable or confirmed case in the last 14 days. Starting in March 11, given the evidence of community spread in the Autonomous Community of Madrid, an RT-PCR test was ordered in all admitted patients exhibiting symptoms compatible with COVID-19. Testing was also performed in children requiring urgent surgery or with household contacts with suspected or confirmed SARS-CoV-2 infection that required admission, who corresponded to the cases excluded from the analysis in our study. Eligibility for confirmatory testing in patients with underlying disease was established on a case-by-case basis. Serology tests became available in late April and performed in venous blood samples. We did not performed tests to confirm the presence of other respiratory viruses routinely. The criteria for admission were the same as before, based on the established protocol of the hospital for the management of each particular condition, independently of the results of tests for detection of SARS-CoV-2 infection.
Definitions- -
Complicated disease: need for haemodynamic support for stabilization (infusion of fluids or vasopressors) or respiratory support (high-flow nasal cannula, non-invasive ventilation, invasive ventilation) or encephalopathy.
- -
Uncomplicated disease: no need of haemodynamic or respiratory support other than supplemental oxygen delivered through a nasal cannula or absence of encephalopathy.
- -
Tachycardia: heart rate above the 90th percentile for age, and extreme tachycardia: heart rate greater than the 99th percentile, based on reference values.13
We collected data on epidemiological, clinical and laboratory variables at the time of admission, the findings of imaging tests during the hospital stay, treatment and patient outcomes. The data were retrieved from health records and, in case of post-discharge complications and outcomes, through phone interviews with the parents. The physician in charge of the patient decided which tests were indicated based on the protocols already established before the pandemic.
Imaging testsChest radiographs were performed in patients with suspected pneumonia based on the clinical manifestations.
Abdominal ultrasound scans were performed in patients with acute abdomen or with haemodynamic instability associated with abdominal pain.
All imaging tests were interpreted by a paediatric radiologist.
Statistical analysisWe made a descriptive analysis of the data with the software SPSS© for Windows (version 20.0). We summarised quantitative data as median and interquartile range and qualitative data as percentages, ranges and interquartile range. We used the Mann–Whitney U test to compare continuous variables and defined statistical significance as a p-value of less than 0.05.
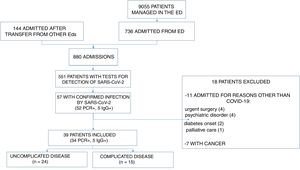
ResultsIn the period under study, 880 patients were admitted to the hospital. A RT-PCR test for detection of SARS-CoV-2 in a nasopharyngeal swab sample was performed in 551, the results of which were positive in 52. Five patients with negative results of the RT-PCR test and a presentation highly suggestive of COVID-19 had detectable levels of IgG antibodies against SARS-CoV-2. We excluded 11 patients because the reason for admission was not directly related to the SARS-CoV-2 infection; all of these patients were asymptomatic or had mild respiratory or gastrointestinal symptoms. We also excluded 7 patients currently undergoing chemotherapy. The final sample included 39 patients (Fig. 1).
Epidemiological characteristicsThe median age was 9 years (range, 12 days–16 years); 12 patients (31%) were aged less than 1 year, 5 (13%) were aged 1–5 years, 9 (23%) were aged 6–10 years and 13 (33%) were older than 10 years; 23 (59%) were male; 27 patients (69%) had contact with a case of COVID-19 in the household, either confirmed (7) or suspected (20). The history of potential exposure was unknown in 12 children.
Clinical and laboratory characteristics, complicated versus uncomplicated courseWe classified patients based on the clinical presentation. We found that 24 patients had uncomplicated disease (Table 1) and 15 had complicated disease (Table 2). The 5 patients with underlying disease had uncomplicated forms of COVID-19. Most children with complicated COVID-19 appeared ill (10/15) and had extreme tachycardia (10/15) in the initial evaluation in the emergency department (ED). An oxygen saturation of less than 94% was infrequent in both groups. Table 3 summarises the clinical characteristics of each case.
Epidemiological characteristics, clinical findings and treatment of patients admitted with uncomplicated disease.
| Clinical presentation | n (N=24) | Median age (range) | Comorbidities (n/total) | Signs and symptoms at the ED (No./total) | Chest X-ray at ED (n/total) | Laboratory abnormalities* (n/total) | Coinfection (n) | Supportive care (n/total) | Pharmacological treatment (n/total) | Length of stay, median (range) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bronchiolitis | 5 | 27 days (12–46) | None | Respiratory distress (5/5)Fever (4/5)Cough (3/5)Food refusal (3/5) | Not performed (3/5)Normal (2/5) | Lymphopenia (1/3)↑LDH (1/1) | B. Pertussis (1) | Oxygen (4/5) | Azithromycin (1/5) | 3 (2–7) |
| Fever without source | 3 | 24 days (23–35) | None | Fever (3/3) | Not performed (3/3) | Normal (3/3) | No | No | No | 3 (3–4) |
| Pneumonia (uncomplicated) | 7 | 4 years (1–12) | Bronchiolitis obliterans (1/7) | Fever (7/7)Cough (5/7)Respiratory distress (5/7)Vomiting (4/7) | Bilateral interstitial infiltrates (3/7)Unilateral consolidation (2/7)Unilateral interstitial infiltrates (1/7)Normal (1/7)** | Lymphopenia (1/7)↑CPR (3/7)↑DD (3/3)↑LDH (3/4)↑PCT (1/4) | Mycoplasma (1)Suspected bacterial coinfection (3) | Oxygen (4/7) | Azithromycin (6/7)Antibiotic (4/7)Corticosteroids (2/7)Salbutamol (3/7) | 4 (2–8) |
| Upper respiratory tract infection | 7 | 12 years (0–16) | Juvenile idiopathic arthritis (1/7)Bone marrow aplasia (1/7)Haemolytic anaemia (1/7) | Fever (6/7)Fever > 7 days (2/7)Cough (4/7)Sore throat (2/7) | Normal (6/7)Not performed (1/7) | Lymphopaenia (2/7) | No | Oxygen (1/7) | Azithromycin (3/7) Antibiotic (4/7)Hydroxychloroquine (1/7) | 2 (1–4) |
| Prolonged diarrhoea | 1 | 54 days | Cow's milk protein allergy | DiarrhoeaVomitingDehydration | Not performed | Normal | No | Fluid replacement therapy | Elemental formula | 3 |
| Abdominal pain | 1 | 12 years | None | Abdominal pain | Not performed | Normal | No | No | Analgesics | 3 |
DD, D dimer; ED, emergency department; LDH, lactate dehydrogenase; PCR, C-reactive protein; PCT, procalcitonin; SatO2, oxygen saturation.
Epidemiological characteristics, clinical findings and outcome of patients admitted with complicated disease.
| Clinical presentation | N (total: 15) | Median age (range) | Comorbidities (n/total) | Signs and symptoms at the ED (No./total) | Chest X-ray at ED (n/total) | Laboratory abnormalities* (n/total) | Coinfection (n) | Supportive care (n/total) | Pharmacological treatment (n/total) | Length of stay, median (range) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shock | 12** | 10 years (6–14) | None | Fever (12/12)Abdominal pain (11/12)Diarrhoea (10/12)Extreme tachycardia (9/12)Ill-appearing (8/12)Rash (8/12)Conjunctival hyperaemia (7/12)Vomiting (6/12)Cough (6/12) | Normal (6/12)***Bilateral consolidation (2/12)Bilateral interstitial infiltrates (2/12)Bilateral interstitial infiltrates with unilateral consolidation (1/12)Unilateral consolidation (1/12) | ↑CPR (12/12)Lymphopaenia (10/12)↑PCT (10/12)↑DD (12/12)↑LDH (8/12)↑ferritin (7/11) | Mycoplasma (1)VHH-6 (1) | PICU(11/12)HFOT (10/12)MV (2/12)Vasopressors (8/12)Blood products (2/12) | Antibiotic (12/12)Hydroxychloroquine (11/12)Heparin (11/12)LPV/RTV (10/12)Azithromycin (9/12)Corticosteroids (11/12)Tocilizumab (6/12)Immunoglobulin (4/12) | 10 (5–22) | Acute heart failure (4/12)Acute respiratory distress syndrome (3/12)Acute kidney injury (2/12)Encephalitis (1/12) |
| Apnoea-bronchiolitis | 1 | 16 days | None | Respiratory distressCoughFood refusalApnoea | Normal | ↑DD | Nosocomial urinary tract infection (Klebsiella pneumoniae) | PICUNIV | AzithromycinAntibioticHydroxychloroquine LPV/RTVCaffeine | 16 | |
| Complicated pneumonia | 1 | 7 months | None | MalaiseExtreme tachycardia FeverRespiratory distressCoughFood refusal | Unilateral consolidation, pleural effusion (1) | ↑CPR↑PCT↑DD | Streptococcus pneumoniae | PICUNIVBlood products Pleural drainage | AzithromycinAntibioticHydroxychloroquine LPV/RTVCorticosteroids | 18 | Necrotising pneumonia |
| Multiple thrombi | 1 | 13 years | None | MalaiseBradycardiaFeverVomitingHeadacheSomnolence | Normal**** | ↑CPR↑DD | No | PICUPICUOxygenBlood products | AzithromycinAntibioticHydroxychloroquine LPV/RTVCorticosteroidsHeparin | 23 | Cerebral venous sinus thrombosisIncreased intracranial pressurePulmonary thromboembolismDeep vein thrombosis |
CRP, C-reactive protein; DD, D-dimer; ED, emergency department; HFOT, high-flow oxygen therapy; LDH, lactate dehydrogenase; LPV/RTV, lopinavir/ritonavir; MV, mechanical ventilation with endotracheal intubation; NIV, non-invasive ventilation; PCT, procalcitonin; PICU, paediatric emergency care unit; SatO2, oxygen saturation.
Epidemiological and clinical characteristics of the patients.
| Uncomplicated disease (n=24) | Complicated disease (n=15) | Total (n=39) | |
|---|---|---|---|
| Median age in years (range) | 2.5 (12 days–16 years) | 9.4 (16 days–14 years) | 9.0 (12 days–16 years) |
| Age distribution – n/total (%) | |||
| <1 year | 10/24 (41.6) | 2/15 (13.3) | 12/39 (30.7) |
| 1 to <6 years | 4/24 (16.6) | 1/15 (6.6) | 5/39 (12.8) |
| 6–10 years | 3/24 (12.5) | 6/15 (40.0) | 9/39 (23.0) |
| >10 years | 7/24 (29.1) | 6/15 (40.0) | 13/39 (33.3) |
| Sex, n/total (%) | |||
| Female | 10/24 (41.6) | 6/15 (40.0) | 16/39 (41.0) |
| Male | 14/24 (58.3) | 9/15 (60.0) | 23/39 (58.9) |
| Comorbidities, n/total (%) | 5/24 (20.8) | 0/15 (0/0) | 5/39 (12.8) |
| Symptoms in the ED, n/total (%) | |||
| Fever ≥38°C | 18/24 (75.0) | 14/15 (93.3) | 32/39 (82.0) |
| Cough | 12/24 (50.0) | 8/15 (53.3) | 20/39 (51.2) |
| Respiratory distress | 13/24 (54.1) | 4/15 (26.6) | 17/39 (43.5) |
| Nasal discharge | 12/24 (50.0) | 2/15 (13.3) | 14/39 (35.8) |
| Food refusal | 7/24 (29.1) | 5/15 (33.3) | 12/39 (30.7) |
| Somnolence | 0/24 (0) | 1/15 (6.6) | 1/39 (2.6) |
| Nausea or vomiting | 7/24 (29.1) | 8/15 (53.3) | 15/39 (38.4) |
| Diarrhoea | 4/24 (16.6) | 10/15 (66.6) | 14/39 (35.8) |
| Abdominal pain | 1/24 (4.1) | 11/15 (73.3) | 12/39 (30.7) |
| Headache | 3/24 (12.5) | 5/15 (33.3) | 7/39 (17.9) |
| Sore throat | 2/24 (8.3) | 2/15 (13.3) | 4/39 (10.2) |
| Rash | 2/24 (8.3) | 8/15 (53.3) | 10/39 (25.6) |
| Conjunctival hyperaemia | 0/24 (8.3) | 7/15 (46.6) | 7/39 (17.9) |
| Apnoea | 0/24 (0) | 1/15 (6.6) | 1/39 (2.6) |
| Dyspnoea | 3/24 (12.5) | 6/15 (40.0) | 9/39 (23.0) |
| Signs at the ED, n/total (%) | |||
| Ill-appearing | 0/24 (0) | 10/15 (66.6) | 10/39 (25.6) |
| Tachycardia | 6/24 (25.0) | 13/15 (86.6) | 19/39 (48.7) |
| Heart rate at P90–P99 | 5/24 (20.8) | 3/15 (20.0) | 8/39 (20.5) |
| Heart rate ≥P99 | 1/24 (4.1) | 10/15 (66.6) | 11/39 (28.2) |
| Oxygen saturation <94% | 4/24 (16.6) | 2/15 (13.3) | 6/39 (15.4) |
| Respiratory distress | 10/24 (41.6) | 10/15 (66.6) | 20/39 (51.3) |
| Imaging tests, n/total (%) | |||
| Chest X-ray in ED | 15/24 (62.5) | 15/15 (100) | 30/39 (76.9) |
| Normal | 9/15 (60.0) | 8/15 (53.3) | 17/30 (56.6) |
| Chest CT scan | 0/24 (0) | 3/15 (20.0) | 3/39 (7.7) |
| Abdominal ultrasound | 2/24 (8.3) | 10/15 (66.6) | 12/39 (30.7) |
| Diagnostic tests, n/total (%) | |||
| RT-PCR for SARS-CoV-2 | 24/24 (100) | 15/15 (100) | 39/39 (100) |
| Positive RT-PCR | 24/24 (100) | 10/15 (66.6) | 34/39 (87.1) |
| Patients with a positive PCR and a previous negative PCR | 1/24 (4.1) | 3/10 (30) | 4/34 (11.7) |
| Serology | 0/24 (0) | 5/15 (33.3) | 5/39 (12.8) |
| Positive IgG test | NA | 5/5 (100) | 5/5 (100) |
CT, computed tomography; ED, emergency department; RT-PCR, real-time reverse transcription polymerase chain reaction; SatO2, oxygen saturation.
Table 4 summarises and compares the laboratory tests performed and the biomarker test results. We found significant differences between groups in the lymphocyte count and the C-reactive protein (CRP), procalcitonin, ferritin and D-dimer levels. In addition, the neutrophil/lymphocyte ratio was higher in patients with complicated disease (p=.001).
Abnormal results of blood test in the emergency department.
| Abnormal test results in ED | Uncomplicated disease (n=24) | Complicated disease (n=15) | p |
|---|---|---|---|
| White blood cells×109/L, median (IQR) | 8510 (5000–12240) | 8060 (7530–10300) | .51 |
| Neutrophils×109/L, median (IQR) | 3420 (2340–5650) | 6720 (6200–9400) | .009 |
| Lymphocytes×109/L, median (IQR) | 2705 (1470–4470) | 750 (350–1800) | .001 |
| Neutrophil/lymphocyte ratio | 1.34 | 12.62 | .001 |
| PCR mg/dL, median (IQR) | 0.5 (0.2–1.7) | 20.6 (15.3–34.2) | <.001 |
| PCT ng/mL, median (IQR) | 0.10 (0.02–0.21) | 5.73 (1.77–14.08) | .001 |
| D-dimer ng/mL, median (IQR) | 490 (230–630) | 3960 (2210–6660) | <.001 |
| LDH U/L, median (IQR) | 285 (203–347) | 305 (272–348) | .27 |
| Ferritin ng/mL, median (IQR) | 77 (20–194) | 686 (255–1392) | <.001 |
CRP, C-reactive protein; DD, D-dimer; ED, emergency department; IQR, interquartile range; LDH, lactate dehydrogenase.
The evaluation in the ED of 30 patients included a chest radiograph, which detected abnormalities in 13 (Table 3). Another 4 patients developed pneumonia during the hospital stay.
An abdominal ultrasound scan was performed in 12 patients, and the most frequent relevant findings were inflammation of the intestinal wall (4/12) and the presence of free intraperitoneal fluid (4/12). The results of the abdominal ultrasound scan were normal in 3 patients.
Course of disease and admission to the paediatric intensive care unitFourteen patients (36%) required admission to the paediatric intensive care unit (PICU): 10 directly from the ED, and 4 from the inpatient ward (all within 36 hours of admission). In the first 5 weeks of the study period, 3 patients were admitted to the PICU with neurologic or respiratory symptoms (1 with multiple thrombi, 1 with apnoea, 1 with pneumococcal pneumonia). The next 2 weeks, 11 children required management in the PICU for suspected MIS-C in the context of COVID-19, most of who presented with gastrointestinal symptoms and shock.
The median length of stay was 4 days (range, 1–23) and the median PICU stay was 5 days (range, 2–21). All children were discharged with no apparent sequelae, and none developed complications thereafter.
DiscussionDespite the global spread of COVID-19, the clinical spectrum of this disease in children is still not well understood. The role played by children in the spread of disease and the mechanism of infection in children also remains unknown. Several studies have described family clusters,7,8,10,14,15 and it seems that it is children who are most frequently infected by adults, probably as a result of the confinement measures imposed to control the spread of the virus. In our study, most children (70%) had a history of exposure to an adult with confirmed or suspected COVID-19 in the household, which suggested that the child was not the index case.
It is widely believed that COVID-19 is milder and less frequent in children compared to adults.16–20 In the period under study, 57 children with infection by SARS-CoV-2 were admitted to one of the two appointed referral hospitals for paediatric inpatient care in the Autonomous Community of Madrid, although they only accounted for 6.4% of total admissions. In the same period, more than 40000 adults were admitted to different hospitals in the same autonomous community with COVID-19.3 This disparity clearly indicates a milder course of disease and lower frequency of COVID-19 in children. Previously published case series reported that 5–67% of infected children had been hospitalised.4,9,14 Based on the current evidence, hospital admission in paediatric patients is more frequent in infants aged less than 1 year and children with underlying disease. In our case series, 12 of the 39 patients were aged less than 1 year (32% of cases), a figure similar to the 40% reported by Cantoni et al. and the 30% reported by Garazzino et al.14 in Italy, although much higher than the percentage described in China (18% in the study by Lu et al.9 and 11% in the study by Dong et al.8) and the 15% reported by the Centers for Disease Control and Prevention (CDC) in the United States.21 Most infants in our hospital had uncomplicated disease with the exception of 1 newborn with apnoea of infancy22 and 1 infant with pneumococcal pneumonia.23 More than half of admitted children were aged more than 6 years, and the median age of children with shock was 10 years. It is not known whether the risk of developing MIS-C in association with COVID-19 is greater in older children.
Admission criteria can vary significantly between facilities. Patients may be admitted as a preventive isolation measure, due to their clinical condition (more severe signs and symptoms) or due to the presence of risk factors for complicated disease. In our hospital, the main criterion for admission was severity. The clinical criteria applied for admission did not differ from the pre-existing hospital protocol for each disease. Most of the patients were previously healthy, and we cannot rule out an increased risk of progression to more severe forms in patients with underlying disease.
Most of our patients presented with fever (87%), which was more frequent compared to the figures reported in China (71%)9 or Italy (36%).15 This could be explained by selection bias, as hospital admission was a criterion for inclusion in our study. In these patients, fever could be indicative of a more severe form of disease.
The symptoms in children with uncomplicated disease were mainly respiratory and similar to those associated with the common cold, flu and flu-like illnesses, bronchiolitis or pneumonia. Some infants aged less than 3 months presented with fever without a source. The clinical manifestations were nonspecific and overlapped with those of other viral or bacterial respiratory tract infections. The results of laboratory tests in this group were mostly normal, save for an isolated elevation of CRP in a few cases. Patients with uncomplicated disease improved with supportive care and were discharged in a few days. Most did not receive any of the drugs proposed for empiric treatment of COVID-19.9,15,24–28
Respiratory infections may also lead to more severe complications, such as apnoea (in very young children), bacterial coinfection or respiratory failure. In our series, one infant had recurrent episodes of apnoea and another developed pneumococcal sepsis and pneumonia with pleural effusion, which progressed to necrotising pneumonia. It is not clear whether the course of disease in the latter case resulted solely from the bacterial infection or from the combination of both infections.
We ought to highlight that 5 weeks after the start of the epidemic outbreak we observed the emergence of a new clinical presentation.10 These patients were older, presented with high fever and reported significant gastrointestinal symptoms (severe abdominal pain, diarrhoea, vomiting) and/or a rash and/or conjunctival hyperaemia. Respiratory symptoms were less frequent in these patients. Most of them presented to the ED with visibly ill appearance and extreme tachycardia. The distinctive feature of this novel presentation was the development of shock shortly after arrival to the ED.29,30. Patients received supportive care and haemodynamic support at the PICU and treatment with antivirals, hydroxychloroquine, antibiotics and anti-inflammatory drugs. All blood cultures were negative. Coronavirus disease 2019 shares some clinical characteristics with other paediatric inflammatory diseases, including Kawasaki disease, myocarditis, toxic shock syndrome, bacterial or intra-abdominal sepsis and macrophage activation syndrome.11 Early detection is crucial. We ought to highlight that these patients exhibited significant elevation of inflammatory markers (CRP, procalcitonin, D-dimer, ferritin) and lymphopaenia, as well as a higher neutrophil/lymphocyte ratio. This ratio has been described as a useful marker in the diagnosis of other illnesses, such as influenza and sepsis.31 There is recent evidence in the literature of an association between the percentage of neutrophils and severe forms of disease in adults, but to date there are no data on the subject for the paediatric population.32 Lactate dehydrogenase did not seem to be a marker of severity in our patients, contrary to the evidence in adults.33 Another salient aspect was that 8 of the 12 patients developed radiological features compatible with pneumonia, despite respiratory symptoms not being the chief complaint at admission.34 Given the clinical manifestations, the results of diagnostic tests and the clustering of cases in a time period that did not coincide with the incidence peaks, we hypothesised that infection by SARS-CoV-2 could trigger an inflammatory response leading to shock. Seven of these patients had a positive result of the RT-PCR tests for SARS-CoV-2, but only 5 had a positive IgG antibody test result, suggesting that this inflammatory response can develop during or after the acute phase.35
There are several limitations to this study. Due to the small sample and the retrospective design, further research is necessary to confirm our findings. Since we only studied patients in our hospital that required inpatient care or with risk factors for severe disease, the data presented here does not reflect the overall incidence of COVID-19 in children and thus cannot be compared to some of the previously published case series. In addition, there was selection bias related to severity that must have affected our findings and conclusions. We were unable to establish the association between infection by SARS-CoV-2 and clinical manifestations in patients in who serology tests were positive but PCR tests were negative. Nevertheless, our study contributes relevant information on the different clinical presentations of COVID-19 in children that may be useful in the early identification of patients at risk of complications.
Our study presents a series of children with infection by SARS-CoV-2 admitted to a single hospital. Patients with isolated respiratory disease and without elevation of classic inflammatory markers had an uncomplicated course of disease. In these cases, empiric use of the drugs proposed for management of COVID-19 was deemed unnecessary. In contrast, older children presenting with fever, gastrointestinal symptoms, elevated inflammatory markers, lymphopaenia and a high neutrophil/lymphocyte ratio exhibited clinical worsening with progression to shock. These patients with complicated disease required intensive care, including supportive care and antiviral and anti-inflammatory medication. Further prospective multicentre studies are required to deepen our knowledge of this new disease in children, such as the EPICO-AEP currently underway in Spain. Its results will contribute to the understanding of the questions we have raised in this article.
FundingThis study did not receive any form of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Storch-de-Gracia P, Leoz-Gordillo I, Andina D, Flores P, Villalobos E, Escalada-Pellitero S, et al. Espectro clínico y factores de riesgo de enfermedad complicada en niños ingresados con infección por SARS-CoV-2. An Pediatr (Barc). 2020;93:323–333.