HIV infection was the main risk of suffering Pneumocystis jirovecii pneumonia (PJP). The clinical-epidemiological characteristics of PJP have currently changed, with there being few studies on this.
MethodsA retrospective observational study was carried out on paediatric patients diagnosed with PJP over a 17 year period in a third level hospital in Spain.
ResultsA total of 23 patients were included, of whom 7/23 (47.8%) suffered a haematological disease, 5/23 (21.7%) a primary immunodeficiency, and 4/23 (17.4%) an HIV infection. Prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) was received by 11/23 (47.8%) patients. All were treated with TMP-SMX and 18/23 (78.3%) with systemic glucocorticoids. There were six (26.1%) deaths, of which one of them (16.7%) suffered an HIV infection. A higher mortality was seen in the non-HIV patients with greater leucocytosis, greater CO2 retention, and a higher heart rate at onset, differences not observed in HIV patients. No differences were found in mortality in relation to the predisposing factor, use of pTMP-SMX, or treatment with glucocorticoids.
ConclusionsPaediatric patients with haematological cancers are currently the main risk group of developing PJP in this age group. No differences were found in mortality between patients with or without HIV infection as predisposing factor. The mortality among non-HIV patients was higher in those that had greater leucocytosis, greater CO2 retention, and increased heart rate at onset. A better prognosis was not seen in patients that received prophylaxis with TMP-SMX prior to the development of the PJP, or in those that received glucocorticoids as part of the treatment.
La infección por VIH era el principal factor de riesgo para padecer neumonía por Pneumocystis jirovecii (NPJ). En la actualidad, las características clínico-epidemiológicas de la NPJ en niños han cambiado, existiendo pocos estudios en este sentido.
MétodosRealizamos un estudio observacional retrospectivo en pacientes pediátricos diagnosticados de NPJ durante 17 años en un hospital de tercer nivel en España.
ResultadosSe recogieron 23 pacientes, de los que 11/23 (47,8%) padecían enfermedad hematológica, 5/23 (21,7%) inmunodeficiencia primaria y 4/23 (17,4%) infección por VIH. Recibía profilaxis con trimetoprim-sulfametoxazol (TMP-SMX) 11/23 pacientes (47,8%). Todos recibieron tratamiento con TMP-SMX y 18/23 (78,3%) glucocorticoides sistémicos. Fallecieron 6/23 pacientes (26,1%), de los que 1/6 (16.7%) padecía infección por VIH. En los pacientes no-VIH con mayor leucocitosis, mayor retención de CO2 y mayor frecuencia cardíaca al debut se evidenció mayor mortalidad, diferencias no objetivadas en pacientes VIH. No se encontraron diferencias en mortalidad en relación con el factor predisponente, empleo de pTMP-SMX ni tratamiento con glucocorticoides.
ConclusionesEn la actualidad, los pacientes pediátricos con neoplasias hematológicas constituyen el principal grupo de riesgo de desarrollar NPJ en este grupo etario. No hemos encontrado diferencias de mortalidad entre pacientes con o sin infección por VIH como factor predisponente. Entre los pacientes no VIH la mortalidad fue mayor en aquellos que presentaron mayor leucocitosis, mayor retención de CO2 y mayor frecuencia cardíaca al debut. No se objetivó mejor pronóstico en pacientes que recibían profilaxis con TMP-SMX previamente al desarrollo de la NPJ ni en los que recibieron glucocorticoides sistémicos como parte del tratamiento.
Pneumocystis jirovecii (P. jirovecii), formerly known as Pneumocystis carinii and considered a protozoan, is now classified as a fungus based on the findings of newly developed gene sequencing techniques.1 It is a ubiquitous microorganism that can only cause disease in humans. It spreads from person to person through contact with respiratory secretions, and its capacity to cause disease depends on the immune status of the host.
Different studies have found a high prevalence of antibodies against Pneumocystis spp in the paediatric population of developed countries, with figures as high as 80% in children under 4 years, proving that primary infection by P. jirovecii in childhood is frequent2,3 and usually asymptomatic.
Immunocompromised patients, especially those with qualitative or quantitative abnormalities in CD4+ T cells, cannot eliminate this pathogen and are at greater risk of developing P. jirovecii pneumonia (PJP).4,5 A decreased CD4+ T cell count is, in fact, the main risk factor for developing this disease.4,6 As would be expected, historically patients with infection by human immunodeficiency virus (HIV) were the main risk group for PJP, experiencing more severe disease and poorer outcomes compared to patients without HIV infection.4 The development of effective antiretroviral therapies and antimicrobial prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) have changed the epidemiology of the disease.4
The diagnosis of PJP in children is usually clinical, as there are no specific laboratory or imaging features of the disease, and microbiological confirmation is performed in bronchoalveolar lavage (BAL) samples, the obtention of which involves an invasive procedure that cannot always be performed in patients with respiratory distress. A possible alternative to BAL in older and cooperative children in good general health is the use of sputum induction. In unstable patients in who it is not possible to perform BAL, it is possible to use nasopharyngeal aspirate, oral lavage or bronchial aspirate (BA) samples, but the sensitivity of these samples is low. Independently of the type of sample obtained, the gold standard for diagnosis of PJP is direct immunofluorescence (DIF),7,8 which offers a high specificity and a variable sensitivity depending on the type of respiratory sample used. Quantitative and qualitative polymerase chain reaction (PCR) techniques offer a higher sensitivity compared to DIF, but they cannot discriminate carriage from active disease.9
There is evidence that early initiation of antibiotherapy and the use of systemic corticosteroid therapy in select cases can improve the outcomes of PJP.10,11
In patients at high risk, prophylaxis with TMP-SMX seems to be the most effective measure for preventing PJP, although it does not completely eliminate the risk of developing the disease.12
The aim of our study was to describe the clinical and epidemiological characteristics and the outcomes of PJP in children managed in a tertiary care hospital in Spain.
Material and methodsWe conducted a retrospective, observational and descriptive study in patients aged less than 18 years given a diagnosis of PJP between January 2000 and March 2017 in a tertiary care children’s hospital in Malaga (Spain), through the collection of data from patient health records.
We defined PJP as any disease with compatible clinical manifestations, imaging features suggestive of PJC and microbiological confirmation through isolation of P. jirovecii in a BAL or BA sample (identified by a positive DIF or PCR test).
We performed a descriptive analysis of the variables under study. We established 2 groups for comparison, patients with HIV infection and patients without HIV infection. In the comparative analysis, we used the chi square test for dichotomous qualitative variables and the Student t test for quantitative variables after verifying the assumption of normality by means of the Kolmogorov–Smirnov test.
The study was approved by the clinical research ethics committee of our hospital, and it was exempt from the need of informed consent as it was a retrospective study that did not involve explicit reporting of data for individual patients.
ResultsWe identified a total of 23 episodes in as many patients. Of this total, 15 (65%) were male, and the median age at the time of the episode was 3.10 years (interquartile range, 0.51−7.20 years). Eleven patients (47.8%) had blood disorders, which was acute lymphoblastic leukaemia (ALL) in 9. Five (21.7%) had primary immunodeficiency disorders (PIDD), which were severe combined immunodeficiencies in 4 (80%) and X-linked agammaglobulinaemia (XLA) in 1 (20%). Only 4 patients (17.4%) had HIV infection. The remaining 3 patients (13.1%) received high-dose systemic corticosteroids (2 mg/kg/day) to treat an underlying disease, including 1 patient with Guillain–Barré syndrome, 1 with mixed connective tissue disease (MCTD) and 1 with haemophagocytic syndrome, the latter 2 of were also treated with other immunosuppressant drugs (Table 1).
Epidemiological characteristics of the sample (N = 23).
| Sex (male), n (%) | 15 (65%) | |||
| Age (years), median (IQR) | 3.10 (0.51−7.20) | |||
| Underlying disease, n (%) | Blood disorder | 11 (47.8%) | ALL | 9 (82%) |
| MDS | 1 (9%) | |||
| Evans syndrome | 1 (9%) | |||
| PIDD | 5 (21.7%) | HLA-II deficiency | 4 (80%) | |
| XLA | 1 (20%) | |||
| HIV | 4 (17.4%) | |||
| High-dose steroid therapy | 3 (13%) | Mixed connective tissue disease | 1 (4.3%) | |
| Guillain–Barré syndrome | 1 (4.3%) | |||
| Haemophagocytic syndrome | 1 (4.3%) | |||
ALL, acute lymphoblastic leukaemia; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; IQR, interquartile range; MDS, myelodysplastic syndrome; PIDD, primary immunodeficiency disorder; XLA, X-linked agammaglobulinaemia.
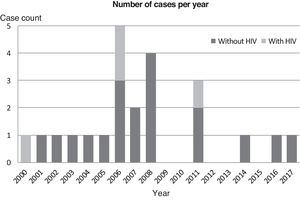
We did not identify any cases of PJP in the subset of patients with HIV in the last 6 years under study (Fig. 1).
Both subgroups (patients with and without HIV infection) were comparable in terms of their epidemiological characteristics (Table 2).
Epidemiological characteristics and clinical and radiographic features.
| Total (N = 23) | HIV+ (n = 4) | HIV− (n = 19) | P | ||
|---|---|---|---|---|---|
| Sex (male), n (%) | 15 (65.2%) | 2 (50%) | 13 (68.4%) | 0.482 | |
| Age (years), median (IQR) | 3.10 (0.51−7.20) | 0.40 (0.22−3.15) | 4.44 (0.66−8.60) | 0.112 | |
| Prophylaxis with TMP-SMX, n (%) | 11 (47.8%) | 1 (25%) | 10 (52.6%) | 0.315 | |
| Fever, n (%) | 21 (91.3%) | 4 (100%) | 17 (89.5%) | 0.544 | |
| Respiratory distress, n (%) | 23 (100%) | 4 (100%) | 19 (100%) | – | |
| Hypoxaemia | 23 (100%) | 4 (100%) | 19 (100%) | 0.629 | |
| Radiographic features | Interstitial infiltrates | 21 (91.3%) | 3 (75%) | 18 (94.7%) | .203 |
| Consolidation | 7 (30.4%) | 1 (25%) | 6 (31.6%) | .795 | |
| Pleural effusion | 1 | 0 | 1 | ||
| Laboratory findings | WBC (cells/mm3) | 11 262 ± 10 131 | 11 972 ± 8346 | 11 084 ± 10 766 | .880 |
| ANC (cells/mm3) | 6190 ± 6424 | 7990 ± 6896 | 5740 ± 6456 | .545 | |
| ALC (cells/mm3) | 3214 ± 4400 | 2645 ± 437 | 3366 ± 4973 | .780 | |
| CRP (mg/L) | 77.4 ± 75.41 | 48.9 ± 59.6 | 84.6 ± 78.9 | .412 | |
| PCT (ng/mL) | 0.64 ± 0.69 | – | 0.77 ± 0.73 | .505 | |
| LDH (IU/L) | 939 ± 671 | 885 ± 248 | 961 ± 792 | .857 | |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CRP, C-reactive protein; HIV, human immunodeficiency virus; IQR, interquartile range; LDH, lactate dehydrogenase; PCT, procalcitonin; TMP-SMX, trimethoprim-sulfamethoxazole; WBC, white blood cell count.
The data correspond to the epidemiological characteristics of the sample (sex, age, previous prophylaxis with TMP-SMX), the presentation of the disease (fever, respiratory distress, hypoxaemia) and the radiological and laboratory findings.
Laboratory values (WBC, ANC, ALC, CPR, PCT, LDH) expressed as mean ± standard deviation.
Eleven patients (47.8%) had received TMP-SMX for prophylaxis before developing PJP. In the subset without HIV, 10 patients (52.6%) received TMP-SMX prophylaxis, of who 9 (90.0%) had ALL and 1 (10%) a PIDD, while 9 (47.4%) had not received prophylaxis, in most cases (6 patients; 66.7%) because PJP was the presentation at the onset of their underlying disease (4 patients with PIDD, 1 with a blood cancer and 1 with haemophagocytic syndrome). The remaining 3 patients without HIV that did not receive TMP-SMX for prophylaxis were receiving high-dose steroid therapy. Of the patients with HIV infection, only 1 had received prophylactic treatment, and in the other 3 PJP was the initial presentation of HIV infection.
The most frequent manifestations at onset were respiratory distress and hypoxaemia, present in every patient. The most frequent radiographic feature was bilateral interstitial infiltration (21/23; 91.3%). Table 2 summarises the laboratory and imaging findings.
When it came to diagnosis, BAL was performed in 22 of the patients (95.6%), with isolation and positive identification of the pathogen in all 22 samples (100%). Fifteen of the 22 samples (68.2%) tested positive with DIF, 11 (50%) tested positive with PCR and 4 (18,2%) tested positive with both techniques. In the patient with MCTD, the diagnosis was made based on a positive PCR test in a bronchial aspirate sample.
All patients received TMP-SMX delivered intravenously at a dose of 20 mg/kg/day, with initiation of treatment a mean of 1 day after the onset of symptoms. Fifteen of the 23 patients (78.3%) were also treated with steroids due to the severity of disease, at a mean dose of 2 mg/kg/day and starting a mean of 2.65 days after the onset of symptoms.
Nineteen patients (82.6%) required admission to the intensive care unit, and 15 of them (78.9%) required mechanical ventilation. Noninvasive ventilation was used in 4 of these 15 patients (26.6%), with a mean duration of 4.5 days, and 2 of these patients eventually required intubation and respiratory support with conventional mechanical ventilation. Conventional mechanical ventilation was used in the other 13 patients requiring ventilation (86.7%), with a mean duration of 7 days, and 8 of them (53.3%) required initiation of high-frequency oscillatory ventilation due to deficient oxygenation, with a mean duration of 3 days.
Six of the patients died (26.1%), of who 5 (83.3%) were patients without HIV infection, although the difference in mortality between patients with and without HIV was not statistically significant. Patients without HIV that died had higher white blood cell counts, higher levels of CO2 in blood and higher heart rates at onset compared to patients without HIV that survived (P < .05). In the subgroup of patients without HIV, mortality was lower in patients that received prophylaxis, with a p-value that neared the threshold for significance (P = .08). We did not find differences in mortality based on the use of steroid therapy or the delay in treatment initiation. We did not find differences in mortality in patients with HIV based on laboratory values, the use of steroid therapy or the use of TMP-SMX prophylaxis (Table 3).
Predictors of mortality.
| Deceased | Survivors | P | |
|---|---|---|---|
| Age (years), mean ± SD | 2.79 ± 2.82 | 5.06 ± 5.27 | .33 |
| Sex (male), % | 50% | 70% | .36 |
| Underlying disease (HIV), % | 16.7% | 17.6% | .96 |
| Prophylaxis with TMP-SMX, % | 16.7% | 58.8% | .08 |
| PCO2 (mmHg), mean ± SD | 85.8 ± 74.45 | 39.4 ± 8.90 | .04 |
| HR (bpm), mean ± SD | 160.8 ± 15.6 | 138.2 ± 17.0 | .02 |
| WBC (cells/mm3), mean ± SD | 22 935 ± 1746 | 5950 ± 5740 | < .01 |
| LDH (U/L), mean ± SD | 984 ± 452 | 781 ± 379 | .40 |
| Steroid therapy (%) | 83.3% | 76.5% | .73 |
| Delay in treatment (days), mean ± SD | 1.17 ± 1.60 | 2.60 ± 3.75 | .38 |
HR, heart rate; LDH, lactate dehydrogenase; PCO2, partial pressure of CO2; SD, standard deviation; TMP-SMX, trimethoprim-sulfamethoxazole; WBC, white blood cell count.
In agreement with previous studies, we found that patients with blood tumours are currently the subset of patients at highest risk of developing PJP.13 The second most important risk group, at a considerable distance, corresponds to patients with PIDDs, especially those with severe combined immunodeficiencies, due to the involvement of the lymphoid cell line. In patients with humoral immunodeficiencies, such as XLA, PJP may develop in the first year of life, probably due to impaired maturation of T cells involved in the cellular response.14 The decline in the incidence of vertical HIV transmission and improved control of the infection in infants born to HIV-positive mothers have led to HIV infection no longer being the main risk factor for developing PJP in developed countries. In our case series, there were no cases of PJP in patients with HIV in the last 7 years.
Although the indications for prophylaxis against P. jirovecii in patient with HIV or cancer are well established, there are no guidelines or recommendations regarding prophylaxis in other risk groups.4,8,10–12,15,16 This, combined with PJP often being the initial manifestation at the onset of the underlying disease, explains why slightly more than half of patients in our case series had not received prophylaxis with TMP-SMX. Of the 12 patients that did not receive prophylaxis, 75% did not have a previous diagnosis of disease increasing the risk of PJP and had onset of the underlying disease with PJP. All 3 of the remaining 3 patients that did not receive prophylaxis were receiving high-dose steroid therapy (for Guillain–Barré syndrome, MCTD and haemophagocytic syndrome) and had not started prophylaxis due to the lack of specific recommendations at the time they developed the disease. As this case series demonstrates, the use of antimicrobial prophylaxis does not completely eliminate the risk of PJP, although the reasons for this remain unknown.
Historically, quicker progression of PJP with more severe respiratory distress and hypoxaemia has been described in patients without HIV compared to those with HIV,4,8,17 a difference we did not find in this case series, probably due to the small number of patients in the subset with HIV group, which hindered the comparison of these groups.
When it came to the diagnosis, it was made through testing of BAL samples in all patients in the series with the exception of a patient with MCTD that could not undergo this procedure due to respiratory insufficiency, in whose case the diagnosis was based on a positive PCR test for P. jirovecii in a bronchial aspirate sample. In most patients in the series, the diagnosis was made by means of DIF, which is the gold standard and offers a sensitivity of 62.5%, similar to the sensitivity reported in patients with HIV.11 In 7 patients (30.4%) the diagnosis was made through qualitative PCR, and 6 of them (85.6%) were patients without HIV, a group in which a lower sensitivity of DIF has been reported on account of lower microbial loads in respiratory samples.18 In addition, 4 of the 7 patients with diagnosis by PCR (57%) were receiving TMP-SMX for prophylaxis, which also reduces the microbial load and therefore the sensitivity of DIF.
All patients in the case series were treated with intravenous TMP-SMX at a dose of 20 mg/kg/day, the first line treatment for PJP recommended by clinical guidelines,8,10,11 which was initiated early. Current recommendations include treatment with oral prednisone or intravenous methylprednisolone in patients with HIV that develop moderate-to-severe PJP, with PJP associated with hypoxaemia or receiving systemic steroids for a different reason, preferably starting within 72 h from initiation of antibiotherapy.8,11 While some studies have found a decrease in mortality associated with the use of steroids for these indications (which is why this recommendation has been extrapolated to patients without HIV meeting the same criteria), our study did not find evidence of a decrease in mortality associated with the use of systemic steroids, probably due to the small number of patients, overall and in the group with HIV, which precluded making meaningful comparisons with the group without HIV. Although every patient in our study presented with hypoxaemia and, based on current recommendations, they should have received steroid therapy, the data collection was retrospective and eligibility for steroid therapy was determined based on the recommendations that existed at the time. In this case series, severe disease might have motivated the decision to initiate adjuvant steroid therapy, which may have been a source of bias toward the null.
In the sample under study, overall mortality was lower compared to other studies on account of a mortality below the 40%–50% that would be expected in patients without HIV.19 The mortality in patients with HIV was similar to the mortality reported in previous studies.8,11 The proportion of patients that died in the series was similar in both groups, contrary to the poorer outcomes in patients without HIV described in the literature.4,15,17 The small sample size and the differences between the two groups may explain these outcomes. These may also explain why our study failed to find differences in mortality based on the history of prophylaxis with TMP-SMX in the overall sample, although in the analysis by groups we found a lower mortality in patients without HIV that had received prophylaxis compared to those that had not. We did not observe this difference in patients with HIV, as only 1 patient in this group, who had a poor outcome, received prophylaxis. In contrast with the previous literature,4,7,16 our study did not find differences in mortality based on other laboratory parameters or the use of steroid therapy, in the overall sample or in either group.
The results of our study must be interpreted with caution, as it had a cross-sectional design that did not allow establishment of causal relationships. Other limitations of the study are its retrospective design, small sample size and the difference in size between the 2 groups, which diminished the power of the analysis to obtain statistically significant results and make meaningful comparisons between groups.
In conclusion, our study revealed that in the paediatric population, PJP currently mainly affects immunocompromised patients without HIV infection, despite the use of antimicrobial prophylaxis. The mortality associated with PJP continues to be high, so it is important that clinicians keep a high level of suspicion, as early initiation of treatment is an important prognostic factor.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martín Pedraz L, Carazo Gallego B, Moreno Pérez D. Características clínico-epidemiológicas de la neumonía por Pneumocystis jirovecii en un hospital de tercer nivel en España. An Pediatr (Barc). 2021;95:4–10.
Previous presentations: The study was presented at the IX Congress of the Sociedad Española de Infectología Pediátrica, 2018, Seville, Spain and the 66th Congress of the Asociación Española de Pediatría, 2018, Zaragoza, Spain.