The International Liaison Committee on Resuscitation (ILCOR) recommendations provide a universal guide of measures to support the transition and resuscitation of newborns after their birth. This guide is expected to be adapted by local groups or committees on resuscitation, according to their own circumstances.
The objective of this review is to analyse the main changes, to discuss several of the controversies that have appeared since 2010, and contrasting with other national and international organisations, such as the European Resuscitation Council (ERC), the American Heart Association (AHA), or the Australian-New Zealand Committee on Resuscitation (ANZCOR). Thus, the Neonatal Resuscitation Group of the Spanish Society of Neonatology (GRN-SENeo) aims to give clear answers to many of the questions when different options are available, generating the forthcoming recommendations of our country to support the transition and/or resuscitation of a newborn after birth, safely and effectively.
Las recomendaciones internacionales del International Liaison Committee on Resuscitation (ILCOR), mediante una revisión exhaustiva de la evidencia disponible en el desarrollo de las medidas de soporte a la transición y de reanimación del recién nacido tras su nacimiento, aportan una guía universal a partir de la cual cada grupo o comité local puede adaptarla a su realidad e idiosincrasia, y elaborar sus propias guías o recomendaciones.
El objetivo de esta revisión es analizar los principales cambios, abordar las controversias generadas desde 2010, contrastarlas con las de otras organizaciones nacionales e internacionales como son la European Resuscitation Council (ERC), American Heart Association (AHA) o la Australian-New Zealand Committee on Resuscitation (ANZCOR). De esta forma, el Grupo de Reanimación Neonatal de la Sociedad Española de Neonatología (GRN-SENeo) consensúa respuestas claras sobre muchas de las preguntas que ofrecen diferentes opciones de actuación, y genera las próximas recomendaciones de nuestro país para el soporte a la transición o la reanimación del recién nacido tras su nacimiento, con seguridad y eficacia.
In October 2015, the International Liaison Committee on Resuscitation (ILCOR)1 (Table 1) updated and published the international consensus recommendations for resuscitation after reviewing the evidence on various controversial issues. Similarly to the previous 2010 recommendations,2 the GRN-SENeo has elaborated a document analysing the main changes occurred since 2010, reviewing the evidence on management options, especially in relation to controversial issues. This has led to the development of the current recommendations for neonatal stabilisation and resuscitation in Spain. These recommendations are an extension of the guidelines published by the ERC,3 AHA,4 and ANZCOR.5 They provide algorithms and specific interventions for each of the sections that include issues that are managed with variable approaches or subject to controversy, and are addressed to professionals involved in the stabilisation of newborns (NBs) in the delivery room.
ILCOR delegations.
| American Heart Association (AHA) |
| European Resuscitation Council (ERC) |
| Heart and Stroke Foundation of Canada (HSFC) |
| Australian and New Zealand Committee on Resuscitation (ANZCOR) |
| Resuscitation Councils of Southern Africa (RCSA) |
| Inter American Heart Foundation (IAHF) |
| Resuscitation Council of Asia (RCA; current members: Japan, Korea, Singapore, Taiwan) |
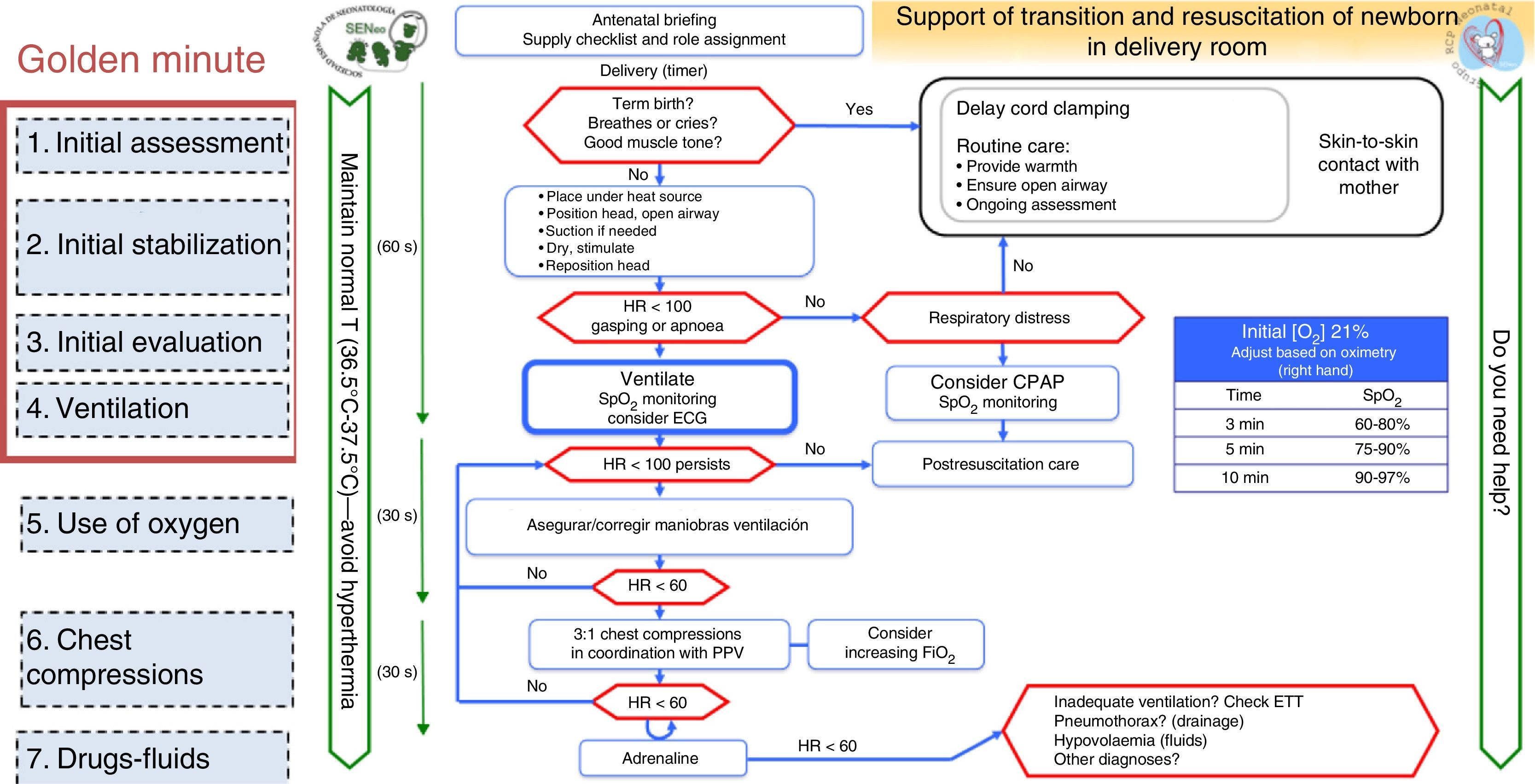
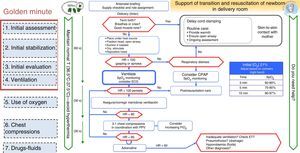
The neonatal resuscitation algorithm of the SENeo consists of a flowchart depicting the sequence of steps to be taken in the resuscitation of a NB in the delivery room (Fig. 1), incorporating parts of different international guidelines.
- •
From the ERC guidelines, we incorporated the maintenance of temperature from birth and the ongoing assessment of whether support is needed at all times.
- •
From the AHA and ILCOR guidelines, we included the “golden minute”, which comprehends initial stabilisation and assessment measures, initiation of intermittent positive pressure ventilation (IPPV) and continuous monitoring by preductal pulse oximetry and ECG.
- •
From the ERC and AHA guidelines, we incorporated: discussing the predicted outcome of the delivery with the family, ensuring that all necessary supplies and equipment are ready for use, and assigning roles to team members (“briefing”).
- •
Visual emphasis on delayed cord clamping in NBs that do not require stabilisation.
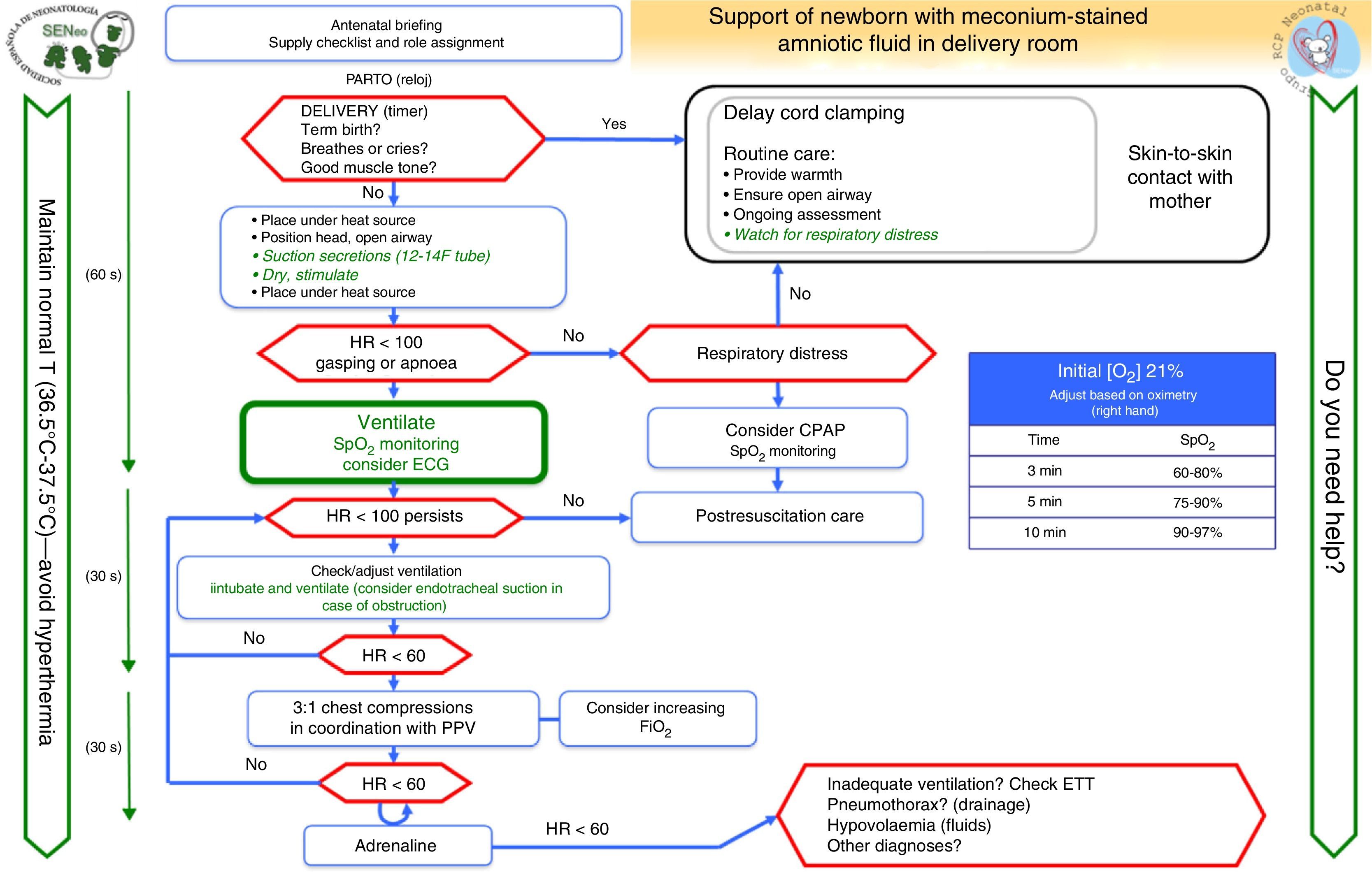
In this article, we review other algorithms developed by the GRN-SENeo on special clinical situations: preterm NBs and NBs with meconium-stained amniotic fluid (MSAF).
Main changes and novelties in ILCOR 2015 compared to ILCOR 2010Support of transitionDuring the transition from intrauterine to extrauterine life, the NB may require routine care or initial stabilisation measures (“support of transition”) that are different from “resuscitation measures”.
Delayed cord clampingDelayed cord clamping (DCC) 30–60s after birth is recommended in term NBs and preterm NBs that do not require resuscitation. As yet there is insufficient evidence to support cord milking as an alternative, and milking is contraindicated in NBs of less than 28 weeks’ gestational age (GA) because there is no evidence on its safety. We emphasise the importance of the “golden minute” mark for completing the initial steps, evaluating the state of the NB and avoiding unnecessary delays in the initiation of ventilation when it is required by the NB.
Body temperature of the newbornThe temperature of nonasphyxiated NBs should be maintained between 36.5°C and 37.5°C after birth and through admission.
Monitoring systemsElectrocardiographic data should be used to estimate heart rate (HR) faster and more accurately to facilitate decision-making.
Neonates born through meconium-stained amniotic fluidRoutine endotracheal intubation and suctioning is not recommended in nonvigorous NBs, and should only be performed for suspected tracheal obstruction. The emphasis should be on initiating ventilation within the first minute of life.
Air/oxygenThe initial use of room air is still recommended in term NBs. Low oxygen concentrations (21–30%) are recommended in patients born before 32 weeks’ gestation if they exhibit laboured breathing. Higher concentrations should be considered if oxygen saturation continues to be inadequate despite adequate ventilation.6
Continuous positive airway pressureThere is growing evidence supporting the recommendation of its use in preterm NBs delivered before 30 weeks’ gestation with spontaneous but laboured breathing to avoid intubation and mechanical ventilation.
Positive end-expiratory pressureThe use of positive-end respiratory pressure (PEEP) is recommended in ventilated preterm NBs.
There are many points of contention in relation to the use of initial supportive measures in the NB: resuscitation at the limit of viability, Apgar score of 0 at 10min from the start of resuscitation as a predictor of morbidity and mortality, respiratory monitoring, capnography, or the clinical use of sustained inflation (SI), among others, and different randomised studies have been proposed.
Support of transition and neonatal resuscitationMost NBs (85%) require only routine care (DCC and skin-to-skin contact with the mother). Under certain pathological conditions, the transition from foetal to neonatal life may be compromised and result in perinatal asphyxia, which in the NB manifests as apnoea, bradycardia and hypotension. Intervention by initiation of stabilisation measures becomes necessary at this point. The overall process constitutes an actual “support of transition” of the NB, which should be distinguished from interventions that restore vital functions or “resuscitation efforts.”
Communication, anticipation and preparation (human and material resources)A novel development is the explicit recommendation of measures such as planning the intervention of resuscitation teams, antenatal briefing, the exploration of family preferences and the involvement of families in the decision-making process based on the prognosis of the patient, which should be made on the basis of objective and updated data.3 This includes redirection of care and, if necessary, initiation of perinatal palliative care (comfort care, psychosocial support and bereavement services). Hospitals must provide care to their maximum capabilities, avoiding putting NBs at increased risk.
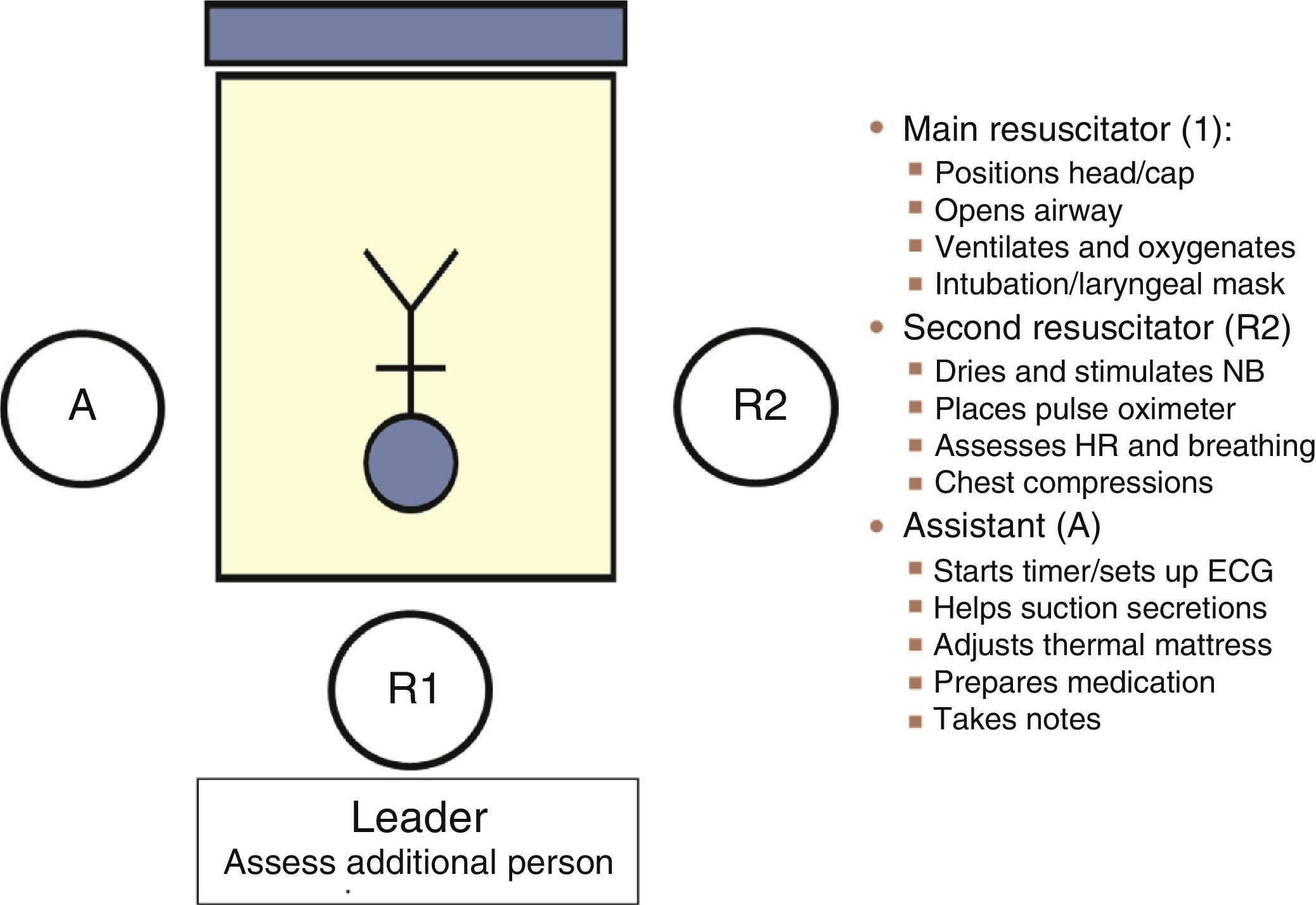
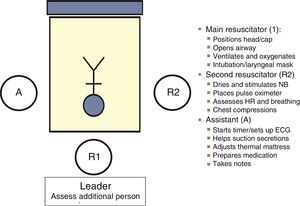
Communication between the obstetrics and neonatal care teams allows the “preparation” of a safe environment: assessment of the situation and risk factors (anticipation), preparation of necessary supplies and equipment (checklist) and human resources (role assignment: coordinator and assistants), followed by an overall assessment of team performance through reflective analysis, or debriefing, for the purpose of optimising team work. The GRN-SENeo has proposed a scheme for role assignment (Fig. 2) in resuscitation teams.7 It is important that team members remain calm, communicate clearly in a closed loop, and express themselves openly (structured SBAR approach). As for the number of members in the resuscitation team, in deliveries with a known increased risk of problems, at least one staff fully trained in neonatal resuscitation should be available and physically present. In high-risk or special situations, the team should consist of at least two resuscitators and one assistant. Resuscitation should be carried out in the delivery room, and the room ready with all the equipment and supplies required to approach any risk situation.1
Fittingly, the ERC 2015 guidelines address planning and care for home deliveries, despite the wide variability between countries. One professional fully trained in resuscitation and another trained in the initial steps of resuscitation should be physically present. Limitations to resuscitation in these deliveries exist and should be made clear to the parents, such as those arising from maternal or neonatal complications or the difficulty in obtaining further assistance should complications arise due to the distance separating the home from a medical facility.3
Initial assessment and stabilisationTemperatureThe temperature of nonasphyxiated NBs should be maintained between 36.5°C and 37.5°C, and this is increasingly important with decreasing gestational age (GA). Skin-to-skin contact with ongoing monitoring of the NB is preferable to any other source of warmth, as it decreases heat losses by 50% to 90% and promotes bonding.
Delayed cord clamping and cord milkingDelayed cord clamping (by 30–60s) is recommended in NBs that do not require resuscitation.2 There is insufficient data to recommend it in NBs that require resuscitation or to recommend cord milking (of 20cm of umbilical cord, 3 times, with NB held at the level of the introitus) as an alternative. The only clinical study published recently on the subject analysed preterm NBs delivered before 32 weeks’ gestation by caesarean section, and found an improvement in systemic blood flow after cord milking compared to DCC that was not observed in NBs delivered vaginally.8 One drawback of DCC studies is that they have not been designed to aerate the lungs prior to clamping the cord. Katheria et al. reviewed the existing evidence and recommendations regarding initiation of ventilation prior to DDC.9 Ongoing clinical trials with initiation of ventilation prior to DCC that will study neurodevelopmental outcomes will guide future recommendations.
Heart rate monitoringThree-lead ECG is the fastest and most accurate method for measuring HR.10 However, early data should be interpreted with caution to avoid unnecessary resuscitation measures, especially in extremely preterm NBs in whom immediate cord clamping prior to initiation of ventilation leads to transient bradycardia (<100bpm) that improves with lung aeration.11 The use of ECG supplements but does not replace pulse oximetry and auscultation for the assessment of oxygen saturation and ventilation, respectively. The ILCOR guidelines1 did not specify an order for the use of different monitoring methods; one possible arrangement would be to assign the following sequence of tasks to the second resuscitator: auscultation, placement of pulse oximeter, and placement of ECG leads to increase monitoring accuracy.
Ventilation and oxygen saturationApproximately 5% of NBs do not achieve adequate spontaneous breathing following stabilisation measures: IPPV should be initiated before 1min of life, so additional time would be available if ventilation continued to be inefficient. Two percent of NBs will require intubation for optimal ventilation.
Following initial stabilisation, the management of the NB is determined based on HR and ventilation (Fig. 1).4 Suctioning of oropharyngeal secretions should not be performed routinely, but only if they seem to cause airway obstruction (using a 8–10F nasogastric tube with a pressure of less than 100mmHg for a maximum of 5s, starting in the mouth and following with the nose). More vigorous suction could delay spontaneous breathing and lead to laryngospasm and vagal bradycardia.
VentilationThe ventilatory values associated with the establishment of an adequate functional residual capacity (FRC) have yet to be determined in clinical practice. Respiratory function monitors and capnographs offer valuable information on tidal volume: they can be used to optimise ventilation, prevent volutrauma or barotrauma and detect adverse events.12 Further research is required to determine adequate tidal volumes for GA so that they can be used as objective paramenters13 to adjust inspiratory pressure and thus minimise lung damage during neonatal stabilisation.
Guidelines have reviewed the various improvements applied in the establishment of adequate FRCs or lung volumes, and there is an open debate on the monitoring of the volumes delivered during preterm NB resuscitation.1,14
On the other hand, the efficacy of SI with prolonged inspiration times in establishing better FRC levels in the transition of NBs with no spontaneous breathing has also been evaluated.15 Our algorithm does not include SI because there is no evidence that it improves long-term morbidity, it is associated with an increased incidence of pneumothorax,16 and there is no standardised procedure for its administration,17 although experiments have demonstrated its efficacy15 (its use is accepted in the context of research) and a clinical trial showed that it decreases the need for intubation and for mechanical ventilation in the first 72h of life.16
We recommend the use of continuous positive airway pressure in term NBs with spontaneous breathing and respiratory distress, although there are not sufficient data on its efficacy and some authors have even warned of the dangers of its excessive use in this age group.
DevicesAccording to the ILCOR,1 the existing evidence does not support recommending a specific ventilation interface (T-tube vs different masks). When intubation is not feasible and after IPPV fails, the use of a supreme laryngeal mask is recommended in the resuscitation of NBs delivered at 34 or more weeks’ gestation. Future studies should assess the effectiveness of its use as the initial interface in reducing the need for intubation.18
Oxygen saturationMonitoring by pulse oximetry should be established in all NBs in whom the need of resuscitation is anticipated based on their clinical condition or persisting central cyanosis.
The ERC3 has set up target oxygen saturation values at the 25th percentile of the normal SpO2 distribution in the first 10min of life,6 similar to the target set by AHA.4 A recent clinical trial found a higher mortality in the subset of newborns delivered before 29 weeks’ gestation resuscitated in room air (16.2%) compared to the group resuscitated with 100% oxygen (6%; P=.013).19 A retrospective cohort study found an increased risk of severe neurologic impairment or death in NBs delivered at 27 or fewer weeks’ gestation following the change in 2006 of the practice of initiating resuscitation with high oxygen concentrations (100%) versus intermediate concentrations (21–40%).20 Following the publication of these recommendations, there have been studies by other groups analysing initial FiO2 values21,22 in the management of moderate to late preterm NBs, and their adjustment after initiation of oxygen supplementation. Due to a lack of clinical trials with representative samples, caution must be exercised in the management of preterm NBs when resuscitation is initiated with room air and in the application of target preductal SpO2 values used to determine adjustments in FiO2. The GRN-SENeo has set the minimum value of SpO2 as above the 15th percentile of the nomograph6 in the first 5min of life.
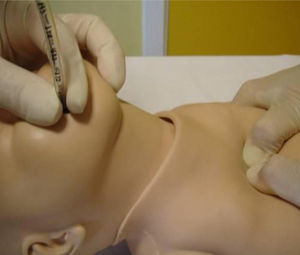
Chest compressionsWhen it comes to chest compression (CC) technique, resuscitation team members should be positioned to the side and inferior to the NB. New approaches such as the thumb-index method have not shown better results than the two-thumb technique (Fig. 3).1
A 3:1 compression/ventilation ratio is still recommended because bradycardia is most commonly secondary to respiratory causes.23 It is still debated whether the oxygen concentration delivered should be increased during CCs. Experiments have found no advantage to the use of high oxygen concentrations in restoring spontaneous circulation, and only one experiment showed improved survival with higher oxygen concentrations, while high concentrations have been associated with neurologic damage.1 If performance of CCs is needed following effective ventilation with a low FiO2, it could be reasonable to increase the FiO2 to optimise oxygenation, adjusting it guided by pulse oximetry once the HR exceeds 60bpm.
Postresuscitation management (temperature, glucose)GlucoseThe current evidence does not suffice to recommend a specific range of glycaemia based on its association to decreased neurologic damage following reanimation of asphyxiated NBs. Sussman et al. have proposed a lower limit of normal of 60mg/dL.24
Studies in neonates25 and experiments in asphyxiated newborn models26 have found an association between hypoglycaemia and adverse neurologic outcomes and decreased survival. Different studies have demonstrated that hyperglycaemia in hypoxic NBs is not associated with adverse outcomes and can even have a protective effect.25,27 However, a recent study that included 528 NBs delivered at 35 or more weeks’ gestation found neurodevelopmental impairment in NBs with higher blood glucose levels, even when they were within the normal range (47–150mg/dL).28 In asphyxiated NBs, IV glucose infusion should be initiated during stabilisation to maintain a blood glucose level between 47 and 150mg/dL.
Temperature at time of admissionIn nonasphyxiated NBs, body temperature is a strong predictor of morbidity and mortality and a quality indicator in all GAs, and especially in preterm NBs. There is evidence of a dose-dependent effect on mortality, with risk increasing by at least 28% with each degree of temperature at admission below 36.5°C.29 The new algorithm calls for maintaining an axillary temperature of 36.5–37.5°C, avoiding hypothermia and hyperthermia.1
Care during induced hypothermiaInternational neonatal resuscitation guidelines, with the exception of those by the AHA,4 do not specify the GA from which the use of hypothermia is indicated. All guidelines agree that clearly defined and consistent protocols developed in randomised clinical trials must be applied in the management of moderate to severe hypoxic-ischaemic encephalopathy. In Spain, this approach has been indicated in NBs delivered at 35 or more weeks’ gestation since 2011 (national guidelines for neuroprotection,30 multicentre national programmes31), and different review studies and clinical trials32 have indicated its use and included NBs delivered at 35 or more weeks’ gestation. Its neuroprotective effects are time-dependent, so passive cooling to core temperatures of 33–34°C should be initiated in patients at risk.33 Active hypothermia should be initiated before 6h of life, but if it is started before 3h it is associated with improved outcomes, especially in the most severe cases.34 This is an advance in that this technique can be applied in countries and settings with few resources.1,3
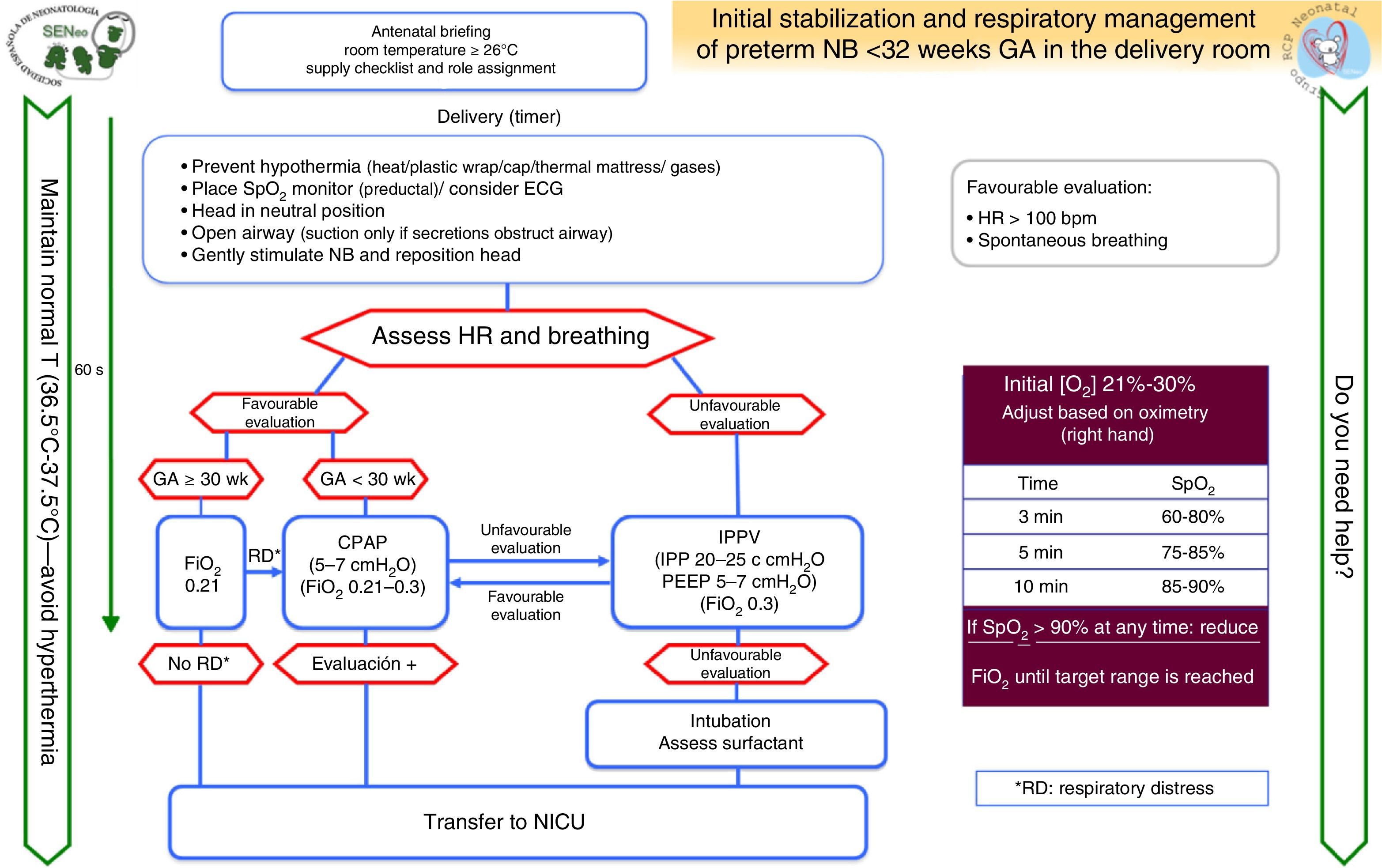
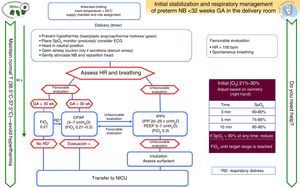
Special clinical situationsPreterm birthFig. 4 shows the algorithm for the resuscitation of preterm newborns less than 32 weeks’ GA developed by the GRN-SENeo.
Prognostic score: the 2015 ILCOR guidelines reviewed the evidence for the use of a convenient prognostic score to predict survival at ages 1 month and 18–22 months compared to only GA in preterm NBs of less than 25 weeks’ GA. There is not enough evidence in support of its prognostic value.35 It would be reasonable to consider other variables, such as the estimated foetal weight, sex, prenatal corticosteroid exposure, oligohydramnios, single vs multiple pregnancy, history of chorioamnionitis, level of care of the medical facility, and clinical practice guidelines.
Temperature: maintaining NB temperature is essential during delivery (ambient temperature of 23–25°C in the delivery room, thermal mattresses, plastic wrap, caps), admission and stabilisation.
Oxygen administration: initial FiO2 with room air is limited to NBs delivered at 30 or more weeks’ gestation and NBs delivered before 30 weeks’ gestation with no respiratory distress, increasing it to 0.3 in the latter if they have respiratory distress. Target preductal SpO2 values increase above the 10th percentile over the first 3min of life, and 60% (15th percentile) is considered a normal value.
Sustained inflation (>5 s): its use is not recommended in preterm NBs without spontaneous breathing, but SI could be considered in specific clinical situations on a case-to-case basis.
PEEP: the use of PEEP is beneficial for preterm NBs with apnoea that require IPPV; more randomised studies are required to assess its benefits.
Cord milking: it is contraindicated in patients born before 28 weeks’ gestation, as there are no data on its safety or long-term beneficial effects.
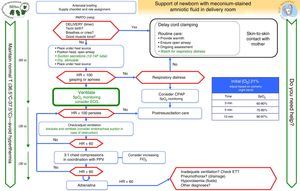
Airway management in infants with meconium-stained amniotic fluid (Fig. 5)The prophylactic endotracheal intubation and suctioning of nonvigorous NBs with MSAF was a controversial issue in the 2010 guidelines. A recent clinical study conducted in India by Chettri et al.36 addressed this issue. In this study, 122 patients were randomly assigned to one of two groups: intubation with IPPV versus endotracheal suctioning followed by IPPV. A similar study conducted in India was presented at the annual conference of the Indian Academy of Paediatrics of 2014.37 Neither study found a difference between the groups. The ILCOR recommendations remain open to three treatment options: “consider in individual cases but do not perform routinely, consider it only if obstruction is suspected or against the recommendation.”1,3,4,38 Our group considers that endotracheal intubation should not be performed routinely in nonvigorous NBs with MSAF, and that the general algorithm should be applied to these NBs. Orotracheal examination and suctioning may be considered if there are signs of airway obstruction by meconium even if ventilation has been initiated and if the resuscitator is experienced in intubation. Repeated intubation is not recommended, and the most important goal is to prevent delays in resuscitation measures, with particular emphasis on initiating ventilation in the first minute of life (Fig. 5).
Ethical considerations. Limit of viabilityFor cases with an uncertain prognosis or at the limit of viability associated with high rates of morbidity and sequelae, development of an individualised care plan is recommended, and whenever parents have been previously interviewed and given consent, the alternative options of initiating resuscitation or withholding it while providing comfort care should be evaluated. When this situation arises in patients born at 23+0–7 to 23+6/7 weeks’ gestation, other perinatal factors should be taken into account.39
In NBs less than 23 weeks of GA or with fatal chromosomal disorders or severe congenital malformations,1 resuscitation should be withheld, initiating comfort and palliative care. In patients born at 24+0–7 weeks’ gestation or later, the initial approach should be active, although it is essential that parental wishes are taken into account in decision making after informing them of the rates of survival found by the SENeo, and providing data on morbidity and mortality for the particular hospital.
Another significant aspect is the discontinuation of resuscitation efforts if the Apgar score continues to be 0 after 10min.40 The ILCOR 2015 guidelines state that the decision to continue or discontinue resuscitation efforts must be individualised, taking into consideration factors such as the delivery setting, material resources, the skills of the team, the availability of therapeutic hypothermia and the wishes of the family.
There is a dearth of evidence and services around the care at the limit of viability and perinatal palliative care, which this Committee considers to be within the scope of our professional competencies. A care protocol is required: warming, analgesia and sedation, ongoing and detailed information and support of parents, allowing parents to spend as much time as they need and want with their child (swaddling, family support, spiritual or religious counselling, etc.) and set up a space that provides privacy.
Conflict of interestsThe authors have no conflict of interests to declare.
Motserrat Izquierdo Renau (Hospital Sant Joan de Déu-Hospital Clínic), Asunción Pino Vázquez (Hospital Clínico Universitario de Valladolid), Eva González Colmenero (Hospital Álvaro Cunqueiro de la Estructura Organizada de Xestión Integrada de Vigo), César W. Ruiz Campillo (Hospital Vall d’Hebron de Barcelona), Dolores Elorza Fernández (Hospital Universitario La Paz, Madrid), Miguel Sánchez Mateos (Hospital Universitario Puerta de Hierro de Mahadahonda, Madrid), Alejandro Ávila Álvarez (Complexo Hospitalario Universitario de A Coruña), Elena García Victori (Hospital Universitario Virgen del Rocío, Seville) and Máximo Vento Torres (Hospital Universitario y Politécnico La Fe, Valencia; School of Medicine, Universidad de Valencia).
Please cite this article as: Zeballos Sarrato G, Salguero García E, Aguayo Maldonado J, Gómez Robles C, Thió Lluch M, Iriondo Sanz M, et al. Adaptación de las recomendaciones internacionales en estabilización y reanimación neonatal 2015. An Pediatr (Barc). 2017;86:51.e1–51.e9.
Appendix A lists the members of the Grupo de Reanimación Neonatal de la Sociedad Española de Neonatología (GRN-SENeo).