In the last decades, allergic diseases have increased exponentially and although pediatric asthma prevalence is stabilizing, it is estimated around 10% in Spain. Not the same with food allergy and anaphylaxis which are clearly increasing, becoming a significant public health problem. Taking into account epidemiological trends, the European Academy of Allergy and Clinical Immunology (EAACI) estimates that in less than 15 years more than half of the European population will suffer from some type of allergic disorder.
The advances in diagnostic methods in food allergy, especially component resolved diagnosis, allow us to know the patient’s sensitization profile and explain possible cross reactivity, anticipate potential risk of food trangressions, and prescribe correct avoidance diet in each patient. Thus, the development of molecular biology and nanotechnology have led to the appearance of new technologies (microarrays) which facilitate the study, specially of the polysensitized patients, allowing allergen immunotherapy (AIT) to be more personalized. The latest advances in the use of biologics are having an impact, not only in disease evolution, but also in quality of life.
En las últimas décadas, las enfermedades alérgicas han aumentado de forma exponencial y aunque parece que la prevalencia del asma en pediatría se está estabilizando, en España se estima en torno al 10%, no ocurre lo mismo con la alergia alimentaria y la anafilaxia que están en claro incremento, constituyendo un problema de salud pública de primera magnitud. Considerando las tendencias epidemiológicas, las predicciones de la Academia Europa de Alergología e Inmunología Clínica (EAACI) estiman que en menos de 15 años más de la mitad de la población europea padecerá algún tipo de alergia.
Los avances en los métodos diagnósticos en alergia alimentaria, sobre todo el diagnóstico molecular, nos permiten conocer el perfil de sensibilización y explicar el fenómeno de la reactividad cruzada, prever el potencial riesgo de las transgresiones alimentarias, e indicar adecuadamente la dieta de evitación en estos pacientes. Así, el desarrollo de la biología molecular y la nanotecnología han llevado a la aparición de nuevas tecnologías (microarrays) que facilitan el estudio, sobre todo de los pacientes polisensibilizados, permitiendo una inmunoterapia específica a alérgenos (ITA) más personalizada. Los últimos avances en tratamientos con biológicos implican un impacto, no solo en la evolución de la enfermedad, sino también en la calidad de vida de los pacientes.
The increase in allergic diseases may be related to many factors, such as the advance in diagnostic methods, the discovery of new allergens, an increased population awareness environmental pollution, changes in dietary habits and the hygiene hypothesis, among others. This increase in prevalence has taken place parallel to the progress in diagnostic and therapeutic techniques.
Based on data from the Alergológica 2015 study, respiratory allergies continue to be the most frequent type of allergy in Spain, with an increase in the diagnosis of allergic rhinitis (AR) and a tendency towards a plateau in asthma. This study found that bronchial asthma accounted for 30.2% of the total medical visits compared to 34.6% in the Alergológica 2005 study. The prevalence of bronchial asthma in Spain is estimated at 10%, with 85% considered extrinsic and 9% poorly controlled. There has also been an increase in the frequency of food allergies compared to 2005. An adverse drug reaction was the reason to seek care in 8.3% of cases. A very small percentage of the population sought care for latex allergy or Hymenoptera venom allergy.1
Allergic rhinitis is the most frequent chronic disease in the paediatric population.2 It affects 25% of the general population of Western Europe, with onset before age 20 years in up to 80% of cases. It is the most frequent presenting complaint in paediatric allergy clinics and 1 of the 10 most frequent reasons for visits to primary care services. In Spain, based on data from the different ISAAC studies, the prevalence of AR is 8.5% in the population aged 6–7 years and 16.3% in ages between 13 to 14 years, with substantial variation between geographical areas due to differences in environmental factors.3 This corresponds to significant health care costs, especially in the management of associated comorbidities, particularly asthma.
In recent decades there has been a sharp increase in food allergies, which has been greater in developed countries. The prevalence peaks at age 1 year at 6%–8% and then declines through the end of childhood, when it reaches values of 3%–4% that remain stable through the years. The prevalence of primary food allergy seems tobe stabilized, but there is evidence of an increase in the frequency of cross-reactivity reactions.4
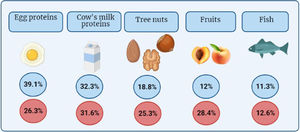
Still, most prevalence studies are based on the results of food sensitivity testing, so the reported prevalence of food allergy may be overestimated. The involved foods tend to be the most frequently consumed foods5 (Fig. 1).
Frequency of the 5 foods most frequently involved in food allergies based on the Allergológica study in the paediatric population1 and changes in frequency in Spain between 2005 (blue) and 2015 (red).
When it comes to drug hypersensitivity, 10% of parents report suspected hypersensitivity to at least 1 drug in their children, although only a few of these cases are confirmed following allergy testing. The drugs most frequently involved are beta-lactam antibiotics, non-steroidal anti-inflammatory drugs and non-beta-lactam antibiotics.6
Changes in diagnostic testingAdvances have been made in the characterization and recognition of symptoms of allergy, resulting in improvements in diagnostic accuracy and treatment. An adequate history-taking and first-line skin prick tests continue to be the cornerstone of diagnosis. Advances in other methods allow the detection of allergen-specific immunoglobulin E (IgE) in cases with a positive skin prick test or a strong suspicion based on the clinical history.
At present, screening methods are available for use at the primary care level,7 such as Phadiatop® and Phadiatop® Infant, qualitative tests that can detect the presence of IgE antibodies against certain allergens (respiratory allergens in the former and food allergens in the latter) in peripheral blood samples. At a later time, antibody levels can be measured through a specific IgE test. Since 2005 a rapid test (20 min) is also available, InmunoCap® Rapid, which offers quick results that are easy to interpret in capillary blood samples obtained from a fingertip prick. This is a qualitative and semiquantitative technique that offers rapid detection of specific IgE against a panel of 10 respiratory and food allergens.8 However, while these methods constitute an advance in the diagnosis of allergy for paediatricians, it is still necessary to ensure that the positive results of testing (sensitization) are consistent with the clinical manifestations (allergy). It is important to avoid eliminating foods from the diet that the patient is sensitised to but can still tolerate.
For paediatric allergists, the next step in the laboratory diagnosis of allergic patients involves molecular testing, or allergy component testing. Natural allergens are complex particles with different types of components, some of which are allergenic molecules, their sensitivity can be assessed independently from the rest of components. Measuring the levels of specific IgE antibodies against individual molecules or allergens offers some advantages compared to testing specific IgE antibodies against the whole allergen. In patients with multiple sensitivities it helps differentiate true sensitization from reactions resulting from cross-reactivity.9 At the same time, it can identify the presence of specific IgE antibodies against molecules associated with greater severity, and thereby can help predict the potential risks involved in exposure to the allergen. It also allows identification of the most relevant allergens, which is very helpful for the purpose of initiating immunotherapy in allergic children .10
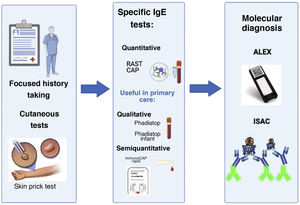
In recent years, diagnostic tests based on microarray analysis, such as the immuno-solid-phase allergen chip (ISAC®) or macroarray analysis, such as Allergy Explorer (ALEX®) have been introduced in specialised allergy care. These are molecular methods that can detect sensitization to a large number of allergen components in very small volumes of serum. They allow more comprehensive diagnosis, although they must be interpreted by an expert due to the risk, once again, of confusing sensitization with allergy11 (Fig. 2).
Relevant advances in the diagnostic approach to allergy. The history-taking and cutaneous tests have been the essential tools for diagnosis from the beginning. In recent years, new diagnostic methods have been developed. The figure shows examples of commercial tests that allow qualitative and quantitative detection of specific IgE as well as developed molecular diagnostic tools (ALEX®, ISAC®) developed more recently to assess sensitivity to a large number of allergen components.
Oral food challenge or provocation tests are the gold standard for definitive food allergy diagnosis. They require controlled and gradual administration of the suspected allergen to confirm or rule out the diagnosis or to assess the development of tolerance.
In the field of respiratory allergies, conjunctival or nasal allergen challenges are performed by local exposure to the allergen with subsequent assessment of the response of each organ through various methods (acoustic rhinomanometry, active anterior rhinomanometry, active posterior rhinomanometry, passive anterior rhinomanometry, peak nasal inspiratory flow and collection of a biopsy sample to assess the cellular response).
The main test used to evaluate respiratory function is spirometry. A positive bronchodilator challenge test supports the diagnosis of asthma. Several studies have corroborated that plasma eosinophil counts are higher in patients with asthma, although the use of this parameter as a marker of bronchial inflammation is controversial. On the other hand, exhaled nitric oxide (FeNO) is a marker of eosinophilic airway inflammation and can be measured in a single expiration with portable devices.
If there are doubts about the allergens involved in bronchial reactions, specific bronchial challenge tests are performed to measure the bronchial response after inhalation of the suspected allergen. Recently, the use of allergen exposure chambers has been introduced in some research facilities.
Changes in the management of allergic diseasesManagement of food allergiesAt present, the standard of care for food allergy is a strict elimination diet,12,13 which carries the potential risk of experiencing accidental reactions requiring safe and effective medical treatment. There have been important advances in the management of anaphylaxis, such as the development of adrenaline auto-injectors in the 1980s, which has facilitated immediate treatment outside the hospital (for example, at school, home or restaurants).
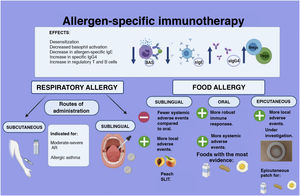
In 1908, researchers reported for the first time the successful ingestion of progressively increasing doses of hen's egg to an adolescent with a previous history of anaphylaxis. Since then, allergen-specific immunotherapy (AIT) has been at the core of research efforts on the subject. Although the underlying mechanism is not fully understood, there is evidence that AIT leads to immunomodulation decreasing basophils and mast cells activation, increasing levels of specific IgG4, reducing levels of allergen-specific IgE and generating regulatory T and B cells.14,15 In general, for both food and respiratory allergens, AIT consists of administration of gradually increasing doses of the culprit allergen (food, standardised allergen extracts) during induction until a specific maintenance dose is reached.16
One of the goals of AIT for IgE-mediated food allergy is desensitization,12 or increasing the response threshold to an allergen, which is achieved in 60%–90% of cases.13,17,18 A more desirable outcome is the absence of reactivity on treatment discontinuation, what is known as sustained unresponsiveness. Sustained unresponsiveness has been described in 27.5%–50% of patients after 2–4 years of treatment.17
The oral route of administration is the most thoroughly investigated to date, mainly for cow’s milk, eggs and peanuts,19 and less frequently for fish, hazelnuts, peach20 and other allergens.13,16,17 Oral immunotherapy is associated with stronger immune response and higher rates of remission.17 Some of the adverse effects described with oral allergen administration are mild allergic reactions, anaphylaxis (6.7%–30.8%)15 and eosinophilic esophagitis (2.7%).15,17
The sublingual route (of which the prime example is immunotherapy for peach allergy) and the epicutaneous route are potential alternatives for allergen administration, with adverse effects that are most frequently local.16,19 However, the safety and effectiveness of these routes in the paediatric population are still under investigation (Fig. 3).
In 2017, the European Academy of Allergy and Clinical Immunology (EAACI) published guidelines on AIT for food allergies, recommending its prescription in patients aged at least 4–5 years for treatment of persistent IgE-mediated allergy to eggs, cow’s milk or peanuts.21 Other scientific societies have also developed clinical practice guidelines to promote a standardised and safe practice of AIT.15,22
In recent years, research in the medical and pharmaceutical fields has focused on innovations in food allergy therapeutics with promising results,23 using modified forms of allergens (powder, high-temperature , food matrix, delivery vehicle) and improving safety and adherence. One important step forward is the authorization by regulatory agencies (Food and Drug Agency [FDA] and the European Medicines Agency [EMA]) of the first standardised product for treatment of peanut allergy (Palforzia®, AR101®).24,25 Also, an epicutaneous patch with peanut, milk and egg (Viaskin®) has been developed and awaits authorization for its commercial distribution.12,17 Research on other strategies is underway (chemical modification of allergens, bacterial plasmid-based DNA vaccines, peptides, alternative routes of administration, probiotics, biologic agents) and at different stages of development.17,26,27
Management of respiratory allergiesThe management of respiratory allergies mainly consists of allergen exposure avoidance measures and pharmacotherapy to control symptoms and underlying inflammation.14,28 However, a significant proportion of patients continue to experience symptoms in spite of receiving the standard of care.28,29
Inhalant allergen immunotherapy was a pioneering empirical treatment first introduced by Noon more than 100 years ago. Since its initial description, specific treatments have been developed, and inhalant AIT is the only disease-modifying treatment available for respiratory allergies.14,29,30 There is significant evidence of its effectiveness in improving AR and allergic asthma in patients aged more than 5 years.14,30,31 Inhalant AIT promotes allergen desensitization, which in turn results in a reduction in both, symptoms and the need for rescue medication.
Its use is currently endorsed by international guidelines in cases of moderate to severe AR and also mild cases to modify the course of the disease (e.g. progression to asthma)28 and for treatment of dust mite-driven asthma32,33 with a duration of 3 years.23,28,30,32,34,35 The subcutaneous and sublingual routes are used most frequently and have exhibited adequate effectiveness and safety (Fig. 3).
Recent advances have produced new modalities of treatment aimed at improving adherence, with a higher effectiveness and safety, through allergen modification (allergoids, recombinant hypoallergenic derivatives), the fusion of allergens with immunomodulators and the creation of peptide transport proteins. Another promising strategy is the use of adjuvants to enhance immunogenicity, of which aluminium hydroxide is used most commonly, although other novel adjuvants can also be used (monophosphoryl lipid A, microcrystalline tyrosine).14,36 Research is also being conducted on alternative routes of administration for which the evidence is still insufficient (intralymphatic and epicutaneous).30
Although many questions remain unresolved, AIT is proving to be a very useful tool.
Biologic agents in the treatment of allergyAdvances in the understanding of the underlying pathophysiology and the phenotype and endotype of allergic diseases have allowed the development of new therapeutic options.
Biologic agents have opened up a novel therapeutic approach,37 chiefly in patients with a poor response to conventional treatment. Initially, biologic agents were used as adjuvant treatment for refractory asthma, since up to 4.5% of children with asthma develop severe disease.37 The evidence of its efficacy in the treatment of other allergic diseases continues to grow.
These agents have a high molecular weight and bind specific molecules (cytokines, receptors), exerting their effects through the modification of activation and signalling pathways of different proteins involved in allergic diseases.
Since biologic agents are selective, they are ideal for the practice of personalised and precision medicine.38 A thorough knowledge of the pathophysiology of the disease to be treated is required to maximise the benefits of these therapies.38
One of the most commonly used biologic agents, omalizumab, has exhibited an adequate effectiveness and safety profile, and proven to be cost-effective compared to the isolated use of conventional medication.38 It was first authorised by the FDA in 2003 and by the EMA in 2005 for use in adults and adolescents aged more than 12 years with persistent severe allergic asthma. The indication for treatment of paediatric patients (age ≥ 6 years) was authorised by the EMA in 2009 and by the FDA in 2016.
Multiple studies have demonstrated that omalizumab achieves a decrease in the use of both inhaled corticosteroids and rescue medication, in emergency department visits and in hospital admissions in addition to improvement in the quality of life of patients with severe asthma.38 It has also been used in combination with AIT, improving the safety of immunotherapy, especially in high-risk cases.
Recent evidence has shown that omalizumab can be useful as adjuvant therapy in patients with severe food allergies undergoing AIT, helping the achievement of desensitization.38 There is also evidence on the benefits in disease activity in moderate to severe chronic spontaneous or inducible urticaria, refractory to the standard first-line treatment, therefore its use is recommended in international guidelines as third-line treatment for this disease.
The first biologic agents approved for children aged more than 6 years were omalizumab and mepolizumab.37 Recently, dupilumab has also been approved for treatment of atopic dermatitis.
Table 1 lists the biologic agents and their corresponding therapeutic indications in allergic diseases.
Biologic agents and their indications in paediatric allergy.
| Biologic agent | Mechanism of action: | Indication: |
|---|---|---|
| Omalizumab | Anti-IgE, binds free IgE. 37 | Children >6 years with persistent severe allergic asthma (fourth step of treatment). |
| Urticaria chronic spontaneous or inducible moderate to severe (third-line). | ||
| Mepolizumab | Anti-IL-5, binds alpha chain of the IL-5 receptor.39 | Children >6 years with severe eosinophilic asthma characterized by frequent and persistent exacerbations in addition to steroid-resistant eosinophilia, who do not respond to omalizumab.40,41 |
| Dupilumab | Acts on the alpha subunit of the IL-4 receptor (anti IL-4Rα), blocking the signalling pathways for both IL-4 and IL-13.42 | Moderate to severe atopic dermatitis in adults and adolescents aged more than 12 years (EMA) |
| Children > 6 years with the same conditions whose disease cannot be controlled with topical medication (FDA).42 | ||
| Severe asthma with peripheral eosinophilia in children aged >12 years. |
Mepolizumab for treatment of atopic dermatitis is currently being investigated, with evidence of only a modest improvement to date, so its use should be reserved for cases refractory to conventional treatment. There have also been promising results with the use of dupilumab for treatment of allergic asthma, with randomised controlled trials showing that it is effective in reducing the incidence of severe exacerbations, improving lung function and controlling asthma in patients receiving inhaled steroids at moderate-to-high doses.
There is no question that biologic therapy has advanced the management of allergic diseases, with an impact not only on the outcomes of disease but also on the quality of life of patients.
Each new advance confirms that in the future, the indication for a biologic agent will also be determined based on biomarkers, endotypes and genetic factors. The integration of these elements in the clinical characterization of patients will result in an increased use of biologic agents, possibly changing traditional management algorithms.38 Other therapies aimed at modifying the different Th2 pathways of the immune system are currently being investigated.
ConclusionsThe prevalence of allergic reactions has increased as advances were made in diagnostic methods and treatment approaches. The continuous search for biomarkers and finetuning of diagnostic tests will allow even more precise and individualised diagnosis and treatment. Advances in the understanding of immunological mechanisms in allergy and tolerance will lead to improvement, development and discovery of novel targeted therapies. For all the above reasons, the outlook of clinical allergy and immunology is bright.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Escarrer-Jaume M, Juan Carlos JB, Quevedo-Teruel S, del Prado AP, Sandoval-Ruballos M, Quesada-Sequeira F, et al. Cambios en la epidemiología y en la práctica clínica de la alergia mediada por IgE en pediatría. An Pediatr (Barc). 2021;95:56.