Although results show an association between the presence of generalised joint hypermobility (GJH) and functional gastrointestinal disorders (FGIDs) in children, they are limited and controversial.
ObjectiveTo determine the association between GJH and FGIDs and the search for risk factors for GJH in girls from a Public Educational Institution of Tuluá, Colombia.
Patients and methodsThe students completed the Rome IV Questionnaire to identify FGIDs. Each girl with a diagnosis of some FGIDs was matched with a healthy control of the same age. Joint laxity was assessed according to the Beighton score and was considered as GJH when it was ≥4. The prevalence of GJH was compared in girls with and without FGIDs.
ResultsOut of a total of 921 girls between 10 and 18 years of age that participated in the study, 219 (23.8%) of them had some FGIDs. The analysis was performed in a total of 169 girls with FGIDs and 169 healthy control girls. There were no significant differences in GJH between girls with and without a diagnosis of some FGIDs (OR=1.12: 95% CI; 0.71–1.77, P=.5838), nor were there any risk factors.
ConclusionIn this study, no relationship or any risk factor was found between GJH and the presence of FGIDs.
En niños, los resultados que muestran asociación entre la presencia de hiperlaxitud articular generalizada (HAG) y trastornos digestivos funcionales (TDF) son limitados y polémicos.
ObjetivoDeterminar la asociación entre HAG y TDF y la búsqueda de factores de riesgo para la HAG en niñas de una Institución Educativa Pública de Tuluá, Colombia.
Pacientes y métodosLas escolares completaron el Cuestionario de Roma IV para identificar TDF. Cada niña con diagnóstico de algún TDF fue apareado con control sano de la misma edad. La laxitud articular se evaluó según el puntaje de Beighton y se consideró HAG cuando fue ≥ 4. Se comparó la prevalencia de HAG en niñas con y sin TDF.
ResultadosEn el estudio participaron 921 niñas entre los 10 y 18 años de edad. Doscientas diecinueve (23,8%) niñas presentaron algún TDF. Fueron analizadas 169 niñas con TDF y 169 niñas controles sanas. No hubo diferencias significativas en la HAG entre las niñas con y sin diagnóstico de algún TDF (OR=1,12 IC95%=0,71-1,77 p=0,5838) ni se presentaron factores de riesgo.
ConclusiónEn este estudio no se logró determinar asociación entre HAG y la presencia de TDF, ni ningún factor de riesgo.
Generalised joint hypermobility (GJH) is defined as increased mobility in several joints caused by an increased laxity of connective tissue.1 In epidemiological studies, the Beighton score is used to identify GJH,2 as it has exhibited a moderate to high repeatability3,4 and is a self-report measure that offers a valid and reliable assessment of joint hypermobility.5 The prevalence of GJH (Beighton score≥4/9) in children ranges from 19.2% to 58.9%6–10 and is associated with female sex,9–11 younger age,2,8,10,12–14 non-white race,10 malnutrition,6,9,13 a greater level of physical activity,6,9 higher socioeconomic status6 and greater maternal educational attainment.9 The prevalence of functional gastrointestinal disorders (FGIDs) as defined by the current Rome IV criteria in children aged 4–18 years ranges from 21.2% to 25.0%, and the most prevalent among them are functional constipation and functional dyspepsia.15,16
In adults, there is evidence of an association between functional gastrointestinal manifestations and GJH, joint hypermobility syndrome (JHS) and Ehlers–Danlos syndrome-hypermobility type (EDS-HT).17–24 Most adults with EDS-HT report gastrointestinal symptoms, most commonly epigastric pain and constipation.22 In children, the evidence supporting an association between the presence of FGIDs and JHS is scarce and controversial.25,26 Most studies that assessed the association between FGIDs and JHS in children and adults were conducted in patients that sought care for gastrointestinal manifestations.18,19,21–24 Only 1 study has analysed the association between FGIDs and JHS in the non-patient population,24 and it did not find an association between JHS and FGIDs. The study has not yet been replicated, and no study to date has analysed this association applying the Rome IV criteria. In light of recent studies that have found an association between certain glycoproteins, such as glycoprotein-tenascin-X, and changes in gastrointestinal function,27 we believe it would be relevant to perform studies to determine whether FGIDs and GJH are associated in the overall paediatric population, which could reflect commonalities in the pathophysiology of connective tissue and functional gastrointestinal disorders, and help elucidate the pathogenesis of FGIDs, which still remains unclear. The potential implications of such an association would transcend the paediatric population, as a proportion of children with FGIDs become adults with FGIDs. The aim of our study was to assess the association between GJH and FGIDs and identify risk factors for GJH in girls attending a public school in Tuluá, Colombia.
Patients and methodsWe conducted a matched case-control study between February 27 and May 16, 2018 in a public school for girls in Tuluá, a city at the centre of the Valle del Cauca, Colombia, with 219138 inhabitants. We started by administering the Spanish version of the Questionnaire on Pediatric Gastrointestinal Symptoms (QPGS) based on the Rome IV criteria, which had been previously validated by our research group28 to female students aged 10 to 18 years. We excluded girls that reported organic disorders in this questionnaire. We then included girls with any form of FGIDs (cases), whom we matched to other girls of similar age and school year that did not meet the criteria for a FGID (controls). Both groups of girls were assessed for GJH by means of the Beighton hypermobility score.2 We also collected data on sociodemographic variables such as race, age (taking into account whether they were school-aged or adolescent), family history of FGIDs and anthropometric measures including weight, height and waist circumference.
Applying the Rome IV criteria, we classified FGIDs as cyclic vomiting syndrome, functional nausea and functional vomiting, rumination syndrome, aerophagia, functional dyspepsia, irritable bowel syndrome, abdominal migraine, functional abdominal pain not otherwise specified, functional constipation and nonretentive faecal incontinence. We classified girls with a Beighton score of 4/9 as cases of GJH. We divided the girls by age group into schoolchildren (10–12 years) and adolescents (13–18 years); by race into mixed, of African descent, white and indigenous; by World Health Organization (WHO) obesity criteria into obese, overweight, with normal weight, moderately underweight and severely underweight; and based on the Vargas classification29 as having or not having abdominal obesity.
We have expressed results as absolute frequencies and percentages or as mean and standard deviation. We analysed the data using Stata 15, using the chi square test or Fisher exact test as applicable. We performed univariate, bivariate and multivariate regression analysis to assess potential effects on the variables of interest and the diagnosis of GJH. We assessed the strength of associations by calculating odds ratios (OR) with their corresponding 95% confidence intervals (CIs). Results with a P-value of less than 0.05 were considered statistically significant.
We obtained signed informed consent from the legal guardians of participants as well as the participants themselves. The study was approved by the Ethics Committee of the Universidad del Valle and the administration of the public school for girls.
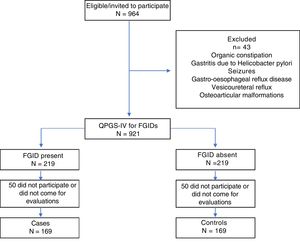
ResultsGeneral resultsWe invited 964 girls aged 12.1±1.4 years (range, 10–18 years) enrolled in years 4–8 of school to participate, and then administered the Spanish version of the QPGS-IV to all for who we obtained a signed informed consent from themselves and their legal guardians. We excluded 43 girls that reported disorders in the questionnaire such as organic constipation, gastritis due to Helicobacter pylori, seizures, gastro-oesophageal reflux disease, vesicoureteral reflux or osteoarticular malformations. A total of 219 girls with some form of FGIDs were recruited for the study and then matched with girls of similar age, sex and school year that did not meet the criteria for any FGID. We assessed joint laxity in both groups of girls using the Beighton score. Fifty girls in each group did not undergo this assessment, so the final sample for analysis consisted of 169 girls with FGIDs (cases) and 169 girls without FGIDs (controls) (Fig. 1).
General characteristicsWe included 169 girls with FGIDs (mean age, 12.2±1.3 years) and 169 without (mean age, 12.4±1.5 years) aged 10–17 years, with a predominance in both groups of girls aged 10–12 years, of mixed race, normal weight and absence of a family history of FGIDs. Table 1 summarises the variables analysed in both groups, between which we found no significant differences. In the total sample of 338 girls, the most common FGIDs in order of decreasing frequency were functional constipation (FC), cyclic vomiting syndrome and functional dyspepsia (FD) (Table 2). We found 21 girls with 2 FGIDs, and the most frequent combinations were FC and aerophagia (1.8%), FC and rumination syndrome (1.5%) and FC and FD (1.2%).
General characteristics of cases and controls.
| FGIDs | Controls | P | |
|---|---|---|---|
| n=169 | n=169 | ||
| Sociodemographic variables | |||
| Age (years) | 12.3±1.4 | ||
| Age groups (years) | |||
| Schoolchildren (10–12) | 92 (54.4) | ||
| Adolescents (13–18) | 77 (45.6) | ||
| Race | |||
| Mixed | 80 (47.3) | 81 (47.9) | .50 |
| African descent | 70 (41.4) | 71 (42.0) | |
| White | 12 (7.2) | 13 (7.7) | |
| Indigenous | 7 (4.1) | 4 (2.4) | |
| Nutritional variables | |||
| Based on BMI | |||
| Malnutrition | 66 (39.0) | 57 (33.7) | .18 |
| No malnutrition | 103 (61.0) | 112 (66.3) | |
| Based on HAZ | |||
| Abnormal height | 2 (1.2) | 0 (0.0) | .25 |
| Normal height | 167 (98.8) | 169 (100.0) | |
| By waist circumference | |||
| With abdominal obesity | 7 (4.1) | 5 (3.0) | .39 |
| Without abdominal obesity | 162 (95.9) | 164 (97.0) | |
| Family-related variables | |||
| FGIDs in the family | |||
| Yes | 3 (1.8) | 4 (2.4) | 0.5 |
| No | 166 (98.2) | 165 (97.6) | |
BMI, body mass index; FGIDs, functional gastrointestinal disorders; HAZ, height for age.
Data expressed as mean±standard deviation or as n (%).
Functional gastrointestinal disorder diagnoses.
| Total | 338 (100.0) |
|---|---|
| No FGIDs | 169 (50.0) |
| Presence of FGIDs | 169 (50.0) |
| Functional nausea and vomiting disorders | 40 (11.8) |
| Aerophagia | 9 (2.7) |
| Functional nausea and functional vomiting | 13 (3.9) |
| Functional nausea | 2 (0.6) |
| Functional vomiting | 11 (3.3) |
| Rumination syndrome | 3 (0.8) |
| Cyclic vomiting syndrome | 15 (4.4) |
| Functional abdominal pain disorders | 31 (9.2) |
| Functional dyspepsia | 14 (4.1) |
| Postprandial distress syndrome | 13 (3.9) |
| Epigastric pain syndrome | 1 (0.3) |
| Irritable bowel syndrome | 9 (2.7) |
| IBS with constipation | 1 (0.3) |
| IBS with diarrhoea | 0 (0.0) |
| IBS with mixed bowel habits | 6 (1.8) |
| Unclassified IBS | 2 (0.6) |
| Abdominal migraine | 4 (1.2) |
| Functional abdominal pain not otherwise specified | 4 (1.2) |
| Functional defecation disorders | 98 (29.0) |
| Functional constipation | 98 (29.0) |
| Nonretentive faecal incontinence | 0 (0.0) |
| 2 FGIDs | 21 (6.2) |
IBS, irritable bowel syndrome; FGID, functional gastrointestinal disorder.
Data expressed as n (%).
Of all girls, 44.1% had GJH based on the Beighton score, corresponding to 45.6% in the FGID group (cases) and 42.6% in the non-FGID group (controls) (OR=1.12; 95% CI, 0.71–1.77; P=.5838). The multivariate analysis did not show any differences between girls with and without GJH (Table 3).
Multivariate analysis of girls with and without GJH.
| GJH | GJH | ||
|---|---|---|---|
| Present | Absent | ||
| 149 (44.1) | 189 (55.9) | ||
| OR | IC95% | P | |
| Sociodemographic characteristics | |||
| Age group | 0.71 | 0.45–1.12 | .1297 |
| Race | 1.20 | 0.76–1.90 | .3959 |
| Nutritional status | |||
| Undernutrition based on BMI | 0.8 | 0.49–1.28 | .3364 |
| Abnormal height based on HAZ | n/a | ||
| Abdominal obesity based on waist circumference | 1.49 | 0.67–3.33 | .2789 |
| Family-related variables | |||
| FGIDs in the family | 0.95 | 0.13–5.71 | .9474 |
| FGIDs | 1.12 | 0.71–1.77 | .5838 |
| Functional nausea and vomiting disorders | 0.89 | 0.41–1.89 | .7543 |
| Aerophagia | 2.69 | 0.55–17.10 | .1563 |
| Functional nausea and functional vomiting | 1.15 | 0.30–4.20 | .8032 |
| Functional nausea | n/a | ||
| Functional vomiting | 0.76 | 0.15–3.16 | .6847 |
| Rumination syndrome | 2.69 | 0.13–160.55 | .4040 |
| Cyclic vomiting syndrome | 2.02 | 0.60–7.20 | .1933 |
| Functional abdominal pain disorders | 0.64 | 0.25–1.53 | .2817 |
| Functional dyspepsia | 0.74 | 0.18–2.61 | .6158 |
| Postprandial distress syndrome | 0.84 | 0.20–3.06 | .7708 |
| Epigastric pain syndrome | n/a | ||
| Irritable bowel syndrome | 0.38 | 0.03–2.11 | .2267 |
| IBS with constipation | n/a | ||
| IBS with diarrhoea | n/a | ||
| IBS with mixed bowel habits | 0.67 | 0.05–4.85 | .6515 |
| Unclassified IBS | n/a | ||
| Abdominal migraine | 1.34 | 0.09–18.95 | .7676 |
| Functional abdominal pain not otherwise specified | 0.44 | 0.008–5.74 | .4811 |
| Functional defecation disorders | 1.09 | 0.64–1.86 | .7154 |
| Functional constipation | 1.09 | 0.64–1.86 | .7154 |
BMI, body mass index; GJH, generalised joint hypermobility; HAZ, height for age; IBS, irritable bowel syndrome; FGIDs, functional gastrointestinal disorder; n/a, not applicable.
Data on the absence or presence of GJH expressed as n (%).
Our study in girls aged 10 to 17 years did not found an association between the presence of FGIDs, identified by means of the Spanish version of the QPGS (Rome IV criteria), and GJH. These results contrast with the findings of Kovacic et al.,25 whose study in a group of children and adolescents with FGIDs managed at a gastroenterology clinic, most frequently with a diagnosis of irritable bowel syndrome or functional dyspepsia, found a 10.03-times greater probability of having GJH (95% CI, 5.26–19.13; P=.001) compared to healthy adolescents in the general population, which led the authors to suggest that an abnormal connective tissue matrix may be involved in the pathophysiology of FGIDs. These data are consistent with our previous findings, although they were obtained in children of both sexes and with a diagnosis of FGID according to the Rome III criteria.9 Our results cannot be compared to the findings of Chelimsky et al.,21 who did not find autonomic dysfunction in girls with benign joint hypermobility syndrome. The cumulative data of the study by Kovacic et al.25 and studies in adults that sought care for gastrointestinal complaints19,20,22–24 suggest that when an association is found between GJH and FGIDs, it is only in patients that seek care because the severity of their comorbidities accounts for the development of FGIDs, and also that there is no organic explanation for this association.
In our sample of girls with and without FGIDs, we found a prevalence of GHJ of 44.1%, a greater proportion compared to 32.9% of the girls included a previous case-control study we conducted in Cali, Colombia (2445281 inhabitants)9 and smaller compared to the prevalence of 56.0% described by Kovacic et al. in the United States.25 These differences in prevalence can be attributed not only to differences in population, ethnicity and regional characteristics, but also to differences in the sex and age of participants and the questionnaire used to identify FGIDs. This overall prevalence of GJH is within the range reported in previous studies at the international level (19.2–60.7%).6–10
In our study, we did not find an association between GJH and any of the analysed variables. As for the association between GJH and FC, which was the main form of FGID found in this sample of girls with a prevalence of 29.0%, Reilly et al.20 found that GJH was much more prevalent in boys with slow transit constipation (STC), which suggests that abnormalities in the synthesis of connective tissue may play a role in the aetiology of STC. However, it is important to take into account that STC is an organic disorder rather than a functional one. Similarly, a study by Kajbafzadeh et al.26 in children with another form of organic disease, in this case urological (urinary dysfunction), found that constipation was more frequent in male patients with GJH compared to those without GJH. In our previous case-control study in children in Cali, Colombia,9 we found that female sex and younger age were associated with GJH. In the general population, different authors have found associations between GJH and higher socioeconomic level,6 female sex,9,10 younger age,2,8,10,12–14 non-white race,8 malnutrition,6,9,13 higher level of physical activity6 and higher maternal educational attainment.10
Some of the strengths of our study is that we used the methodology proposed for FINDERS (Functional International Digestive Epidemiological Research Survey Group) applied to our previous study with the former version of the Rome criteria,11 in which girls were evaluated by staff trained on the performance of the Beighton score and the use of questionnaires in Spanish validated for diagnosing FGID in a context that allowed us to obtain data on children in the community without the selection bias inherent in the profile of individuals that seek medical care. Some of the weaknesses are that our study only included female students and was conducted in a single public school without comparison with a private school, that we did not ask about other potential comorbidities that could be useful to assess the impact of GJH and FGIDs, and that these were all girls that attended school and did not go to a health care facility.
In conclusion, our study in girls aged 10–17 years enrolled in one public school did not find an association between GJH and the presence of FGIDs identified by means of the Spanish version of the QPGS based on the Rome IV criteria. We were also unable to identify any risk factor for GJH among the variables analysed, which suggests the need for different types of studies enrolling children managed in specialty clinics able to assess whether connective tissue laxity is associated with the presence of severe FGID and the presence of comorbidities, which leads to seeking medical care.
Conflicts of interestThe authors have no conflicts of interest to declare.
This article is part of a doctorate dissertation in Clinical Medicine and Public Health developed at the Universidad de Granada, Granada, Spain. Research area: Pathophysiology of medical-surgical diseases.
Please cite this article as: Velasco-Benítez CA, Ruiz-Extremera Á, Saps M. Estudio de casos y controles sobre hiperlaxitud articular generalizada en escolares con trastornos digestivos funcionales según los criterios de roma IV en español. An Pediatr (Barc). 2019;91:401–407.