At present, the therapeutic armamentarium for cancer awaits the irruption of new tools emerging in the field of cellular immunity. The chief advance is chimeric antigen receptor (CAR) T-cell therapy, a medicinal product in the group of advanced gene therapies designated as a breakthrough therapy by the American Society of Clinical Oncology in 2017.1 It consists in the ex vivo genetic engineering of autologous T cells harvested by means of apheresis to enable the expression in the cell surface of a specific chimeric membrane receptor capable of recognising a target antigen in tumour cells.2
In November 2018, the National Plan for the Management of Advanced Therapies in the National Health System: CAR T-cell medicinal products3 was approved in Spain, with the main objective of organizing the use of CAR T-cell therapies for management of 2 diseases: B cell acute lymphoblastic leukaemia (ALL) in patients aged up to 25 years, and diffuse large B cell lymphoma, in both cases for treatment of relapsing or refractory disease.
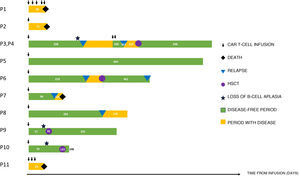
In this letter, we describe the experience of our hospital from December 2017 to September 2021. A total of 21 infusions were performed in 14 patients. Seven were infusions of tisagenlecleucel (Kymriah®, Novartis) for an approved indication (CD19+ B-cell ALL), 3 tisagenlecleucel in the context of a clinical trial, and 11 infusions of CAR T-cells made in house as part of an academic trial. Of these patients, 2 received 6 infusions of allogeneic CART45RA-NKG2D cells, 2 received 5 infusions of autogenous dual-targeted bispecific CD19/CD22 CAR T cells, both through expanded access with the prior approval of the Agencia Española del Medicamento y Productos Sanitarios, as they were not eligible for tisagenlecleucel or had relapsed after its administration (Table 1) (Fig. 1).
Summary of characteristics of patients treated with CAR T-cell therapy.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAR T | NKG2D | NKG2D | Dual CD19/CD22 | Tis-cel | Tis-cel | Tis-cel | Tis-cel | Tis-cel | Tis-cel | Tis-cel | Dual CD19/CD22 |
| Underlying disease and indication of CAR T-cell therapy | MPL relapse after second haplo-HSCT | B-ALL recurrence after second haplo-HSCT | B-ALL combined relapse after CD19 CAR T-cell | B-ALL combined relapse after 2 lines of treatment | B-ALL relapse after URD HSCT | B-ALL relapse after URD HSCT | Refractory B-ALL | Infant B-ALL relapse after HSCT | Ph-like B-ALL relapse after HSCT | B-ALL in second medullary relapse after second-line treatment with | B-ALL in relapse after CD19 CART cell and haplo-HSCT |
| Bridge therapy | No | No | TIT | PRED + VCR + TIT | PRED + ARA-C + PEG-ASP + VCR + VBL + TIT | DEX + VCR + PEG-ASP + TIT | DEX + VCR + ARA-C + CFM | DEX + VCR + PEG-ASP + TIT | PRED + VCR + PEG-ASP + CFM + ARA-C + TIT | TIT | PRED |
| % blasts in bone marrow pre-CAR T-cell | 50 | 87 | 0.01 | 0.01 | 30 | 60 | 5.5 | 84 | 0.01 | 1.3 | 4.56 |
| Lymphodepletion regimen | None | FluCy | FluCy600 | FluCy | FluCy | FluCy | FluCy | FluCy | FluCy | FluCy | FluCy |
| Days elapsed from prescription to infusion | 21 | 20 | 112 | 43 | 42 | 48 | 49 | 41 | 55 | 54 | 20 |
| Infused dose of CAR T cell/kg | 5 × 107 (5 aliquots) | 1 × 107 (1 aliquot) | 3 × 106 (2 aliquots) | 2.8 × 106 | 1.7 × 106 | 2.3 × 106 | 2.6 × 106 | 2.7 × 106 | 3.28 × 106 | 2.5 × 106 | 4.2 × 107 (3 aliquots) |
| CRS (ASBMT grading) | No | Grade 2 | Grade 1 | Grade 1 | Grade 1 | Grade 3 | Grade 3 | Grade 1 | No | Grade 1 | No |
| ICANS (ASBMT grading) | No | No | No | No | No | Grade 3 | No | No | No | No | No |
| HLH | No | No | No | No | No | Yes | Yes | No | No | No | No |
| Infection | No | IFI | No | No | No | No | TB, adenovirus | No | No | No | No |
| Response at day 30 | Progression | Progression | CR | CR | CR | CR | CRi | CR | CR | CR | Progression |
| Loss of B-cell aplasia | N/A | N/A | Yes | No | No | No | No | No | Yes | Yes | No |
| Relapse (location and phenotype) | Progression | Progression | Combined (medullary and CNS CD19+ | Combined (medullary and CNS) CD19+ | No | Medullary CD19– | Medullary CD19– | Lymph node CD19+ | No | No | Medullary CD19– |
| Time from CAR T-cell infusion to relapse/loss of B-cell aplasia (months) | N/A | N/A | 1.4 | 7 | N/A | 7,3 | 3,3 | 9,4 | 1,9 | 2,33 | N/A |
| Management after relapse | Palliative care | Palliative care | Haplo-HSCT | Dual CD19/22 CAR T | No | Haplo-HSCT and ribociclib | Palliative care | Haplo-HSCT | Haplo-HSCT | Haplo-HSCT | Palliative care |
| Follow-up (months) | 2.2 | 2.4 | 12.6 | 23.26 | 22.13 | 15.36 | 4.3 | 15.3 | 12.53 | 4.86 | 1.96 |
| Current status | Deceased | Deceased | Alive in CR | Alive in CR | Alive in CR | Alive in relapse | Deceased | Alive in CR | Alive in CR | Alive in CR | Deceased |
The table excludes the 3 patients treated in the CCTL019C2202 clinical trial.
ARA-C, cytarabine; B-ALL, B-cell acute lymphoblastic leukaemia; CAR, chimeric antigen receptor; CFM, cyclophosphamide; CNS, central nervous system; CR, complete remission; CRi, complete remission with incomplete haematologic recovery; CRS, cytokine release syndrome; DEX, dexamethasone; FluCy, fludarabine (30 mg/m2 × 4 doses) and cyclophosphamide (500 mg/m2 × 2 doses); FluCy600, fludarabine (30 mg/m2 × 4 doses) and cyclophosphamide (600 mg/m2 × 2 doses); HLH, haemophagocytic lymphohistiocytosis; HSCT, haematopoietic stem cell transplantation; ICANS, immune effector cell-associated neurotoxicity syndrome; IFI, invasive fungal infection; MPL, mixed-phenotype leukaemia; N/A, not applicable; PEG-ASP, pegylated asparaginase; Ph-like, Philadelphia chromosome-like; PRED, prednisone; TB, tuberculosis infection; tis-cel, tisagenlecleucel; TIT, triple intrathecal therapy; URD, unrelated donor; VBL, vinblastine; VCR, vincristine.
All the apheresis procedures were completed without complications followed by successful engineering of the medicinal product. Following infusion, there were 8 episodes of cytokine release syndrome, 1 of immune effector cell-associated neurotoxicity syndrome and 2 of haemophagocytic syndrome. These adverse events led to admission of 4 patients in the intensive care unit. However, there were no deaths associated with CAR T-cell therapy toxicity.
The rate of remission achieved by the treatment was 100% in the case of tisagenlecleucel and 50% in the case of dual-targeted CD19/CD22 CAR T-cell therapy. Three of the patients treated with tisagenlecleucel experienced loss of B cell aplasia in peripheral blood. This occurred in the first 6 months in 2 patients, which led to early performance of haematopoietic stem cell transplantation (HSCT) used as a consolidation strategy, after which there has been no known recurrence of disease. In the remaining patient, who exhibited loss of B-cell aplasia past the 6-month mark, the approach was watchful waiting, with eventual diagnosis of CD19+ medullary relapse. This shows that loss of B-cell aplasia is a predictor of relapse, especially in the first year.
In the group of patients with B-cell ALL that achieved complete remission with tisagenlecleucel, we observed 4 relapses, 3 medullary and 1 extramedullary (lymph node recurrence). The approach to patients that relapse after CAR T-cell therapy is controversial. In our series, 2 patients were rescued successfully with a second HSCT and 1 other with dual- 19/22 CAR T-cell therapy. However, we believe it necessary to identify the patients that would require HSCT for consolidation after tisagenlecleucel therapy, even in the presence of B-cell aplasia, to prevent future recurrence. In addition, monitoring chimerism of lymphocyte lineages after HSCT in patients that received tisagenlecleucel could be an alternative to monitoring of B-cell aplasia as a screening approach to identify the risk of relapse and initiate preventive therapies such as adoptive immunotherapy.4
Although in vitro studies of NKG2D-CAR products have shown a good safety profile for treatment of blood and solid tumours,5 there is a dearth of clinical data and, in our experience, the clinical outcomes are not encouraging.
The evidence to date suggests that CAR T-cell therapy offers a good safety profile and efficacy for paediatric patients with recurrent or refractory B-cell ALL.1 However, loss of CAR T-cells and relapse continue to be frequent and have unfavourable outcomes. More thorough research is required on the outcomes of CAR T-cell therapy in real-world clinical practice and on the optimization of these treatments, and protocols for consolidation strategies after CAR T-cell therapy need to be developed. All of this would achieve an increase in survival in this group of patients and expand the indications of this therapeutic approach to other types of paediatric tumours.6
FundingWe thank the Fondos FEDER (FIS) PI18/01301, as well as the Instituto de Salud Carlos III (ISCII) and the Fundación Cris contra el Cáncer (http://criscancer.org) for their support.
Please cite this article as: Galán-Gómez V, González B, Vasserot I, Mirones I, Pérez-Martínez A. Resultados preliminares tras la instauración del programa con medicamentos CAR-T en leucemia aguda linfoblástica en un centro pediátrico. An Pediatr (Barc). 2022;97:131–134.