Blood culture (BC) is the gold standard when a bacteraemia is suspected, and is one of the most requested microbiological tests in paediatrics. Some changes have occurred in recent years: the introduction of new vaccines, the increasing number of patients with central vascular catheters, as well as the introduction of continuous monitoring BC systems. These changes have led to the review and update of different factors related to this technique in order to optimise its use. A practice guideline is presented with recommendations on BC, established by the Spanish Society of Paediatric Emergency Care and the Spanish Society for Paediatric Infectious Diseases. After reviewing the available scientific evidence, several recommendations for each of the following aspects are presented: BC indications in the Emergency Department, how to obtain, transport and process cultures, special situations (indications and interpretation of results in immunosuppressed patients and/or central vascular catheter carriers, indications for anaerobic BC), differentiation between bacteraemia and contamination when a BC shows bacterial growth and actions to take with a positive BC in patients with fever without source.
El hemocultivo (HC) es el método diagnóstico de elección ante la sospecha de bacteriemia, siendo una de las técnicas microbiológicas más solicitadas en pediatría. Diversos cambios han acontecido en los últimos años como la introducción de nuevas vacunas, el aumento creciente de pacientes portadores de catéteres vasculares centrales, o la irrupción de los sistemas automáticos de procesamiento de los HC. Dichos cambios han propiciado la revisión y la actualización de los distintos aspectos relacionados con esta técnica con el fin de optimizar su uso. Se presenta una guía práctica sobre recomendaciones acerca de la extracción, el procesamiento y la interpretación de los HC elaborada por la Sociedad Española de Urgencias de Pediatría y la Sociedad Española de Infectología Pediátrica. Tras revisar la información científica disponible, se presentan una serie de recomendaciones para cada uno de los siguientes apartados: indicaciones en Urgencias, técnica de extracción, transporte y procesamiento de la muestra, factores a tener en cuenta en situaciones especiales (indicaciones e interpretación de resultados en el paciente inmunodeprimido y/o portador de catéter vascular central, indicaciones de HC para anaerobios), diferenciación entre bacteriemia y contaminación ante un HC con crecimiento bacteriano y actitud a tomar ante un HC positivo en el paciente con fiebre sin foco.
Blood culture (BC) is one of most frequently ordered microbiological tests in paediatrics. The detection of bacteraemia is essential, as it is associated with a considerable morbidity and mortality. On the other hand, differentiating between bacteraemia and a contaminated culture can lower the costs associated to BC. It is also necessary to restrict the indication of BC to those patients at increased risk of bacteraemia.
In recent times, various developments have led to shifts in the epidemiology of bacteraemia. The introduction of new vaccines has led to significant changes in the incidence and aetiology of bacteraemia. Furthermore, the increase in the number of immunocompromised patients, patients with intravascular catheters and patients receiving broad-spectrum antibiotics has promoted the emergence of bacteraemias by microorganisms that used to be a rare cause of infection or considered contaminants in the past. On the other hand, the advent of automated BC systems has significantly reduced the waiting times for results and increased the number of positive cultures.
In light of the new situation, the Working Group on Infectious diseases of the Sociedad Española de Urgencias de Pediatría (Spanish Society of Paediatric Emergency Care) and an ad hoc group of the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectology) have reviewed and updated the existing literature on this microbiological test.
Indications for drawing blood cultures in the emergency settingThere is no consensus on the indications for drawing BCs in paediatric emergency settings. There is little data on focal infections, and when it comes to patients with fever without source (FWS), the new situation following the introduction of new vaccines has led to changes in their management.1–6 We have divided our recommendations into three groups according to the existing evidence on the diagnostic yield of BC (Tables 1 and 2).
Indication for collection of BC samples in patients with fever without source in an emergency setting.
| Collection is always recommended in the following situations: |
| Suspected sepsis/septic shock/toxic shock |
| Suspected meningococcaemia |
| Investigation of prolonged fever |
| Infants aged <3 months with fever without source (FWS) |
| Patient admitted for parenteral antibiotherapy due to suspected bacterial infection |
| Fever in immunocompromised patients |
| Patient presenting with fever after returning from travel to a tropical area |
| Consider collection in: |
| Infants aged 3–36 months with FWS >39°C and incomplete pneumococcal vaccination |
| Routine collection is not recommended in: |
| Infants >3 months with FWS with good general health that have completed pneumococcal vaccination |
Indications for collection of BC samples in patients with focal infection in the emergency setting.
| Group A. Collection is recommended if any of the following are suspected or apply: |
| Bacterial meningitis |
| Endocarditis |
| Osteoarticular infection (arthritis/osteomyelitis) |
| Severe pneumonia |
| Complicated pneumonia (necrosis, abscess, pleural effusion, empyema, pneumatocoele, etc.) |
| SSTI: deep SSTIs (pyomyositis, necrotising fasciitis) and complicated superficial SSTIs (secondary to trauma, surgical site infection, ulcer, burn or bite, immersion wound, pericatheter, prosthetic material, needing surgery, extensive involvement, or suspected ecthyma gangrenosum) |
| Urinary tract infection in infants <3 months |
| Infants <3 months with any localised infection that requires admission |
| Infections in immunocompromised patients and patients with intravascular catheters |
| Group B. Consider collection in case of: |
| Pneumonia requiring hospital admission |
| Urinary tract infection in patient requiring admission |
| Suspected complicated appendicitis/peritonitis |
| Complicated infection in the ENT region (mastoiditis, suspected para/retropharyngeal abscess) |
| Group C. Do not perform routine collection in: |
| Uncomplicated superficial SSTIs |
| Pneumonia that does not meet the criteria for admission |
| Pyelonephritis in healthy children without hospital admission |
SSTI, skin or soft tissue infection; ENT, ear–nose–throat.
Group A: Drawing a BC is strongly recommended.
The recommendation for drawing a BC is unanimous:
- –
Clinical suspicion of sepsis.
- –
Focal infections with a prevalence of bacteraemia greater than 10%.
- –
Patients with FWS and a risk of occult bacteraemia (OB) greater than 1.5%.
Group B: Drawing a BC is advisable in:
- –
Focal infections carrying a risk of bacteraemia of 1–10%.
- –
Patients that require admission to receive parenteral antibiotherapy.
- –
FWS with a risk of OB between 0.5% and 1.5%.
Group C: Routine collection of a BC sample is not recommended in:
- –
Focal infections carrying a risk of bacteraemia of less than 1%.
- –
FWS with a risk of OB of less than 0.5%.
- –
Infants aged less than 3 months: The overall rate of OB ranges between 2% and 2.2% and rises to 4.4% in association with a positive urine culture.2 At present, performance of BC is recommended in all infants aged less than 3 months and with FWS equal to or greater than 38°C.2,3
- –
Infants aged 3–36 months: The rate of OB in children aged 3–36 months with FWS greater than 39°C and in good general health ranges between less than 0.5% and 0.9%, depending on their pneumococcal vaccination status.1,5 A study conducted in Spain showed differences in the rate of OB depending on the number of doses of vaccine received, and was significantly higher in patients that had received none or one dose compared to patients with two or more doses.5 These findings have led to the inclusion of pneumococcal vaccination status in the protocols for the management of febrile infants. Other studies have shown similar rates in the groups of vaccinated and unvaccinated individuals.1 The Group of Experts did not reach a unanimous opinion on whether management should vary based on pneumococcal vaccination status, although most agreed with the recommendations presented in Table 1.
- –
Degree of fever: Fever higher than 40°C has been associated with a greater probability of a serious bacterial infection (SBI) in infants aged less than 3 months; in older children, the correlation between the degree of fever and SBI is not clear. The duration of fever or the response to antipyretics are not good predictors for OB and must not direct changes in the management of children with FWS.7
- –
Immunocompromised patients: Performance of BC is necessary due to the high rates of OB in these patients.
- –
Prolonged fever: In patients with FWS of more than 7 days’ duration, performance of BC is recommended.
- –
Parenteral antibiotherapy: In patients with FWS in whom parenteral antibiotherapy is indicated, a BC must be drawn prior to initiating treatment.
- –
Fever in returned travellers from tropical areas: Drawing a BC and testing for malaria are indicated in children presenting with fever after returning from a tropical area or endemic region.
A BC must always be drawn in children with manifestations of sepsis, septic shock or toxic shock.3,8
Indications in patients with localised infections (Table 2)- –
Meningitis: In patients with suspected meningitis, BC has a high diagnostic yield and should always be performed.9
- –
Pneumonia: The prevalence of bacteraemia in children with community-acquired pneumonia ranges between 2.1% and 7%, and exceeds 10% in cases of complicated pneumonia and 20% in cases of empyema.10 Drawing a BC is recommended in all patients requiring admission due to their clinical condition or in whom complicated pneumonia is suspected.
- –
Skin and soft tissue infection (SSTI): In a recent study on uncomplicated superficial SSTIs, none of the patients had bacteraemia, compared to 12.5% of patients with complicated infections.4 Thus, a BC should only be drawn under specific circumstances (Table 2).
- –
Osteoarticular infections: Drawing of blood for culture is indicated prior to initiating antibiotherapy due to its high diagnostic yield (>50%).11
- –
Urinary tract infections (UTIs) with fever: The rate of bacteraemia depends on age, reaching up to 20% in newborns and less than 5% in children older than 3 months. Thus, a BC should be drawn in infants aged less than 3 months with suspected UTI and in older children requiring intravenous therapy.2,12
- –
Endocarditis (IE): Extraction of sample for BC is indicated in any child with suspected IE.
- –
Intraabdominal infections: In case of suspected acute peritonitis, extraction of a sample for BC is recommended, although there is not sufficient data on its diagnostic yield and a sample of peritoneal fluid/exudate is the gold standard for culture.
- –
Complicated infection of the ear-nose-throat (ENT) region: The option of drawing a BC should be considered in complicated infections of the ENT region requiring admission.
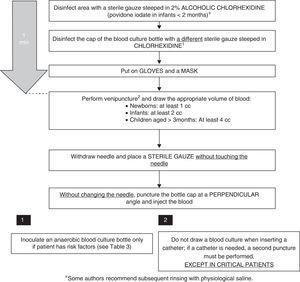
Several factors in the collection process can lead to a higher yield and a lower rate of contamination in BC samples:
- –
Sample volume: While in neonates both the volume of the sample and its dilution in relation to the culture medium are important,13 in older children volume is the most important factor to increase the yield. It has been reported that each additional millilitre of blood that is cultured increases the rate of isolation by 0.6–4.7%. Inoculation of an inadequate volume—either excessive or insufficient—is a frequent cause of false negatives in BC, and has been described as occurring in more than 50% of submissions.14 Generally speaking, the recommendation for the culture media used most frequently in paediatrics is a blood-to-medium dilution of 1:5. As most bottles for paediatric BC contain 20mL of medium, ideally 4mL of blood would be collected, but it is difficult to extract this amount in newborns and infants, in whom collection of 1 and 2mL of blood, respectively, is considered adequate. Adult bottles should be used for the collection of 10mL of blood or more.
- –
Number of BCs: Serial drawing of BCs in paediatric patients has proven to be of little use,15 except in those that are immunocompromised.
- –
Selection of culture medium: Performance of anaerobic blood cultures in addition to aerobic cultures does not increase the yield of BC, save in patients with risk factors for bacteraemia caused by anaerobic pathogens.16 The Microbiology laboratory should be consulted when an atypical or fastidious pathogen is suspected.
- –
Disinfection of the venipuncture site: Some studies show that alcoholic chlorhexidine at concentrations higher than 0.5% has superior antimicrobial activity than other products, with its effects peaking one minute following application.17,18 There is evidence that the use of 2% alcoholic chlorhexidine in patients aged more than 2 months is safe, but its use in younger infants has not been researched, so povidone iodate should be used in the latter age group. Some of the authors in the Group of Experts recommend a subsequent rinse with physiological saline, and the literature does not provide evidence to support or refute this recommendation. Another important aspect is the use sterile gloves during the procedure.19
- –
Timing and site of puncture: It is recommended that the BC sample be collected preferably from the antecubital region, and if the clinical condition of the patient permits it, to delay collection to the time the fever starts to spike.
- –
Collection technique: The sample must not be collected from a previously inserted catheter (except when catheter-related infection is suspected). There is evidence that this increases the rate of BC contamination by a factor of 2–3,15,20,21 so the sample should always be collected by venipuncture, as opposed to from an intravascular catheter. Some authors recommend the use of disposable masks when BC is performed.19,22 The time the needle is removed is also often critical. Applying pressure with a cotton ball at the site of venipuncture to prevent formation of a haematoma is a widespread practice. At times, this gesture is performed so quickly that the cotton touches the needle, increasing the risk of contamination. We recommend the use of sterile gauze for this purpose, always placing it after the needle has been fully removed.
- –
BC bottle: Disinfecting the cap of the BC bottle reduces the number of contaminated samples. However, changing the needle prior to inoculation of the bottle does not seem to be useful, and it is associated to accidental punctures, so it is not recommended.23 It is important to remember to always inoculate the BC bottle first.
We have developed a step-by-step protocol for drawing BCs based on the reviewed literature (Fig. 1).
Transport and processing of blood culturesFollowing inoculation, BCs should be submitted to the laboratory as quickly as possible so they can be placed in the incubator of the automated system. If this is not possible and transport is delayed, BCs for automated systems should be stored at room temperature away from direct sunlight and for no longer than 18h to maintain bacterial viability. At present, the methodology for processing BCs is established and universally accepted, and is performed with a high degree of standardisation.24 The time needed for detection of growth depends on the involved microorganism and the bacterial concentration of the inoculum.
Once growth is detected in a BC bottle, a Gram stain is performed to determine bacterial morphology, and based on the findings subcultures in the most suitable media and an antibiogram are performed.
Blood cultures in special circumstancesImmunocompromised patientsThere is enough evidence to support the recommendation of drawing BCs in immunocompromised patients with a temperature of 38°C or higher, or even with a sustained low-grade fever.25,26 The real challenge arises if the isolated microorganism is part of the normal flora of the patient, for example, coagulase-negative staphylococci, viridians group streptococci, Corynebacterium species, Propionibacterium acnes, Bacillus species and some Clostridium species.26,27 In these cases, it is hard to differentiate contamination from true bacteraemia. One factor to consider is the number of BCs in which the same organism is isolated. Assuming that not all samples have been collected from the same contaminated source, the isolation of the same microorganism in more than one culture increases the probability of a true bacteraemia. Conversely, the presence of only one positive BC in two or more serial draws performed in a short period of time would be more suggestive of contamination.26,27 In any event, there is no definitive method to differentiate contamination and true bacteraemia, and in each case the interpretation of the results of BC will ultimately rest with the clinician.
Patients with central venous cathetersIn children with central vascular catheters or port-a-cath systems, the simultaneous drawing of two or more BC samples is recommended, with at least one central venous catheter sample and one venipuncture sample. The isolation of the same bacterium in both BCs increases the probability of a true bacteraemia, as well as the therapeutic recommendation of removing the colonised catheter.15,27 Faster rates of bacterial growth in the BC correlate to higher probabilities of true bacteraemia.28
Indication of anaerobic blood cultureWe recommend that anaerobic cultures be ordered only in situations with a high degree of clinical suspicion17,29,30 (Table 3).
Indications for drawing anaerobic blood culture samples.
| Sepsis originating in the abdomen |
| Sepsis of cutaneous origin with potential involvement of anaerobic pathogens (severe oral mucositis, bites, crush wounds or sacral ulcers) |
| Nosocomial sepsis following abdominal or trauma surgery |
| Sepsis with refractory hypotension |
| Fever originating in the teeth |
| Chronic infections (sinusitis, osteomyelitis) |
| Immunocompromised patients |
Contaminated BCs may lead to unnecessary admissions, delays in diagnosis, administration of antibiotics and performance of additional BCs or other tests. For these reasons, when a BC exhibits bacterial growth it is essential that true bacteraemia and contamination are differentiated early on. We proceed to present the main factors to consider.
Isolated microorganismThe epidemiology of bacteraemia is subject to constant changes that are related to antibiotic use, vaccination, geographical differences and emerging microorganisms.31–33 Currently, the most frequently involved pathogens are: (a) during the neonatal period: Streptococcus agalactiae, Escherichia coli, Klebsiella species and Listeria monocytogenes, and (b) starting from age 30 days: Streptococcus pneumoniae, enterobacteria, Staphylococcus aureus and Neisseria meningitidis (Table 4). In immunocompromised children, Pseudomonas aeruginosa and Candida species should also be considered. In recent years, advances in molecular biology methods have contributed to the identification of other aetiological agents, such as Kingella kingae or Ureaplasma species.31
Microorganisms most frequently involved in bacteraemia and in contamination of blood cultures.
| Pathogenic microorganisms |
| Streptococcus agalactiae |
| Streptococcus pneumoniae |
| Staphylococcus aureus |
| Neisseria meningitidis |
| Escherichia coli |
| Salmonella spp. and other enterobacteria |
| Streptococcus pyogenes |
| Contaminating microorganisms |
| S. epidermidis and other coagulase-negative staphylococci |
| Viridians group streptococci |
| Corynebacterium spp. |
| Propionibacterium spp. |
| Bacillus spp. |
| Nonfermenting Gram-negative bacilli (other than P. aeruginosa) |
The bacteria in the commensal skin flora are the most common contaminants leading to false-positive results in healthy children (table 4). However, we must take into account that they can be the cause of nosocomial or health care-related infections, especially in catheterised preterm infants and immunocompromised children with intravascular catheters.
Time to positivityWith the advent of automated systems, there has been significant improvement in the time to detection of growth of pathogens that cause bacteraemia. The time to positivity (TTP) for 95% of these pathogens is less than 24h. While clinical criteria still prevail, the rapid detection of positive cultures is a factor to consider in the decision-making process. When the growth corresponds to contaminating organisms, the TTP is markedly longer, exceeding 24h in most cases.34
In children with central venous catheters, the difference in the TTP between BCs from catheter samples and from venipuncture samples allows the diagnosis of the focus of the bacteraemia (isolation ≥2h earlier in catheter samples indicates that the catheter is the focus of the bacteraemia). In order to assess the differential TTP, samples must be collected simultaneously and the same volume of blood inoculated in each of the BC bottles.35 The TTP has also proven useful for the differentiation between contamination and bacteraemia in BCs positive for coagulase-negative staphylococci; a TTP of 15h or less usually indicates a clinically significant result, while a TTP of 22h or more is suggestive of contamination.36
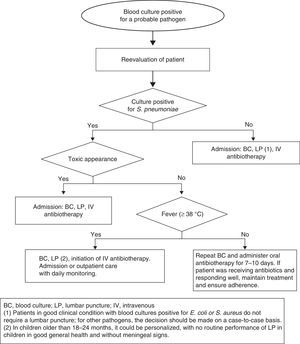
Approach to uncontaminated positive blood cultures in healthy children with fever without sourceThe Department of Microbiology usually reports positive growth in BCs between 24 and 36h from sample collection or even earlier, informing of the type of microorganism involved based on Gram staining. If the sample was collected from a patient with FWS, the patient is often at home. Patients in whom true bacteraemia is suspected must be reevaluated and managed according to their age, clinical condition, persistence of fever, and type of microorganism that was isolated.37
- –
Infants aged less than 3 months: A sepsis workup should be performed along with hospital admission and administration of empirical antibiotherapy based on the preliminary microbiology results.38
- –
Children aged 3–36 months (Fig. 2).
- –
Persistent fever: If the source is not identified, a new BC must be drawn, performance of a lumbar puncture strongly considered in children aged less than 18 months, and parenteral antibiotherapy administered. In patients with good general health, after antibiotic treatment initiation, therapy can be maintained in the home for 7–10 days.
- –
Afebrile patients: These patients may be treated with oral antibiotics at home under close monitoring. Performance of another BC prior to initiating antibiotherapy should be considered, as the risk of persistent bacteraemia is 9%.39 The initial antibiotherapy regimen should be:
- -
Amoxicillin: 80–90mg/kg/day in 3 doses (maximum, 3g/day).
- -
Patients allergic to penicillin will be prescribed treatment with a macrolide or cefuroxime axetil (30mg/kg/day) if there is no history of severe anaphylactic reaction.
- -
In cases in which appropriate antibiotic treatment has already been initiated, the treatment will continue, and the decision to draw a follow-up BC made on a case-to-case basis. All cases will be closely monitored for 7–10 days by a primary care paediatrician.
Bacteraemia caused by other pathogensThe published studies on bacteraemia caused by organisms other than S. pneumoniae suggest that administration of oral antibiotherapy does not prevent severe bacterial infection even in afebrile children in good general health. Furthermore, the risk of meningitis is greater in patients with bacteraemia caused by N. meningitidis. Consequently, hospital admission is required in patients with a BC positive for N. meningitidis, Hib, S. aureus, Gram-negative bacteria or other pathogens. A lumbar puncture is recommended not only in patients with clinical manifestations of meningitis, but also in patients with a BC positive for N. meningitidis and infants aged 3–6 months with bacteraemia caused by group B Streptococcus. Conversely, a lumbar puncture is not routinely required in children in good general health aged more than 3 months with cultures positive for E. coli or S. aureus.
- –
Duration of antibiotic treatment: Until the results of the antibiogram become available, antibiotic treatment will be initiated based on the suspected diagnosis and the sensitivity of the microorganism in each medium. The recommended duration is 7–10 days for uncomplicated bacteraemias, and the customary duration if the focus of infection is found.
- –
Indication for extraction of a follow-up BC sample: The benefits of follow-up BCs for establishing the microbiological cure of bacteraemia have not been proven.40 It is recommended that follow-up BCs are performed 48–96h after initiation of antibiotic treatment in the following cases:
- -
Bacteraemia by S. aureus (especially MRSA) or multidrug-resistant Gram-negative bacilli.
- -
Candidaemia.
- -
Persisting fever or absence of clinical improvement after 48–96h of appropriate antibiotic treatment.
- -
Recurrence of fever.
- -
Suspected IE.
- -
The authors have no conflict of interests to declare
Please cite this article as: Hernández-Bou S, Álvarez Álvarez C, Campo Fernández MN, García Herrero MA, Gené Giralt A, Giménez Pérez M, et al. Hemocultivos en urgencias pediátricas. Guía práctica de recomendaciones: indicaciones, técnica de extracción, procesamiento e interpretación. An Pediatr (Barc). 2016;84:294.