Necrotising enterocolitis (NEC) is the most frequent gastrointestinal emergency in preterm infants. Despite advances in neonatal care, the mortality associated to advanced NEC exceeds 30%. The plain radiograph continues to be the gold standard of diagnosis and staging, despite the nonspecificity of early features (dilated bowel loops) and the substantial variability observed in the interpretation of other signs such as intramural gas.1 Abdominal ultrasound is mainly used as a second-line tool in the evaluation of these patients. In this article, we present 2 cases in which NEC was diagnosed at an early stage by means of point-of-care ultrasound (Fig. 1).
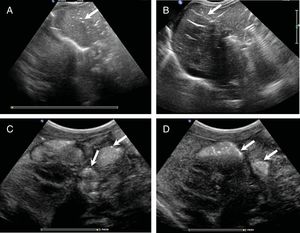
Abdominal ultrasound scan revealing portal gas and intestinal pneumatosis: Note the presence of numerous air bubbles in the peripheral branches of the left portal vein, in the form of dots (A) or as streaks in a branching pattern over the right lobe of the liver (B) Liver pattern resembling an air bronchogram. Bubbles are trapped in the narrow peripheral portal vessels and can be visualized, even if gas is not moving during performance of the ultrasound. Intestinal pneumatosis appearing as isolated foci (C) or clustered foci in the bowel loop walls (D). (Appendix B, supplemental material, video.)
Case 1. Infant born at 35 weeks’ gestation admitted with severe hypoglycaemia secondary to congenital hyperinsulinism and a haemodynamically significant patent ductus arteriosus. On day 6 post birth, the infant developed abdominal distension but remained in good general condition, with normal bowel movements and blood test results. An abdominal plain radiograph was performed that detected nonspecific bowel loop dilatation. The point-of-care ultrasound scan performed by the paediatrician revealed the presence of portal gas and several foci of intestinal pneumatosis. The ensuing diagnosis of NEC prompted discontinuation of enteral nutrition and initiation of antibiotherapy. The patient deteriorated rapidly in the hours that followed, developing tachycardia, poor perfusion, respiratory distress and elevation of acute phase reactants. The patient was intubated and treated with fluids and dopamine. At 48h, the abdominal radiograph evinced the presence of intestinal pneumatosis, portal gas and a fixed bowel loop. Thereafter, the patient exhibited slow but sustained improvement, and did not require surgery (Fig. 1).
Case 2. Infant born preterm at 28 weeks’ gestation with a favourable transition to extrauterine life that was under high-flow oxygen therapy and had attained full enteral feeding. On day 21 post birth, blood was detected in the stools and there was an increase in the frequency of episodes of apnoea. In the physical examination, the abdomen was soft without distension and was not tender on palpation. The blood tests were normal, without elevation of acute phase reactants. Point of care abdominal ultrasound revealed the presence of gas in the liver parenchyma and the hepatic portal system, along with dilatation and enlargement of a bowel loop and isolated foci of intestinal pneumatosis. These findings led to discontinuation of enteral nutrition, placement of a central venous catheter at the left brachiocephalic vein and initiation of antibiotherapy, low-dose dopamine and parenteral nutrition. In the hours that follow, the patient experienced respiratory deterioration, passed another 3 bloody stools and developed abdominal distension, while the follow-up blood tests detected a low white blood cell count, anaemia and elevation of acute phase reactants. The plain radiograph showed nonspecific bowel dilatation. The clinical outcome was good, and the patient did not require surgery.
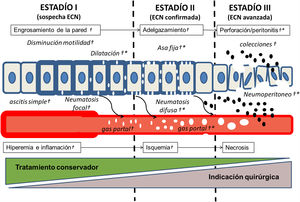
Intestinal pneumatosis and portal gas are the most specific radiologic signs of NEC, and their presence confirms the diagnosis. Ultrasonography allows detection of very small volumes of portal gas and pneumatosis that would not be visible in a plain radiograph.2 Thus, ultrasonography allows an earlier but equally accurate diagnosis of NEC. In addition, unlike radiography, ultrasound allows an objective assessment of the changes of the intestinal wall (thickening and hyperaemia followed by thinning and ischaemia), which makes it possible to intervene in anticipation of perforation (Fig. 2).3 It also performs better than radiography in the diagnosis of ascites or collections, and it is comparable for diagnosis of pneumoperitoneum.4 For all the above reasons, the value of diagnostic classifications based on radiographic findings is being questioned, and an increasing number of experts recommend the use of sonography as the first-line imaging method for diagnosis and follow-up of NEC.5 It would be reasonable to assume that diagnosis in the early stages of disease before ischaemia develops in the intestinal wall could improve outcomes, preventing progression to perforation and the need of surgery. In this sense, we believe that point-of-care ultrasound is perfectly suitable for NEC. Specifically, it allows quick and easy detection of gas in the hepatic portal system and the liver parenchyma. This technique has been proposed for screening of NEC in preterm infants with abdominal symptoms of unclear aetiology, as in many cases, like we observed in our patients, the gas was detectable before systemic involvement, which allowed the differentiation of NEC from other entities with a sensitivity of 82% and a specificity of 96%.6 Detection of portal gas in the ultrasound scan is not independently associated with the need of performing surgery, which supports the hypothesis that this feature develops in the early stages of disease.3 In conclusion, we believe that selective screening of NEC by point-of-care ultrasound is a strategy that could improve early diagnosis and should be investigated in specific research studies.
Natural history and radiographic and sonographic features of enterocolitis: modified Bell stages with the corresponding changes in blood vessels and the intestinal wall. Onset with inflammation and increased permeability of the intestinal wall, leading to hyperaemia and increased density of mesenteric vessels. The intestinal wall thickens, the bowel loops become dilated and ileus develops. As the intestinal wall is invaded by gas-producing bacteria, pneumatosis develops, first focal and localised and eventual diffuse. Leakage of air in the blood vessels leads to visualization of gas in the portal system, first in the peripheral vessels of the liver and, as the amount of air increases, in the main branches of the hepatic portal vein. As the disease progresses, blood flow decreases, ischaemia develops in the intestinal wall, which starts to thin, and finally there is necrosis and perforation leading to bacterial invasion of the peritoneum and the systemic circulation. Perforation may be contained, with formation of complex ascites and fluid collection, or pneumoperitoneum may develop. (†) marks signs detectable by ultrasound and (*) the signs detectable by radiography. Note that focal pneumatosis and portal gas are visible on ultrasound at very early stages of disease (stage I).
Please cite this article as: Oulego-Erroz I, Terroba-Seara S, Alonso-Quintela P, Jiménez-Gónzález A, Ardela-Díaz E. Ecografía a pie de cama en el diagnóstico precoz de la enterocolitis necrotizante: ¿una estrategia para mejorar el pronóstico. An Pediatr (Barc). 2020;93:411–413.