In recent years, the use of point-of-care ultrasound (PoCUS), understood as the bedside ultrasound examination of the patient by the physician in charge, has been increasing in paediatric patients for the investigation of pulmonary, pleural and diaphragmatic disease, following its use in adult patients, in whom the technique has been developing for more than a decade, and whose ultrasound semiotics can be safely extrapolated to the paediatric age group. The unossified costal cartilage and sternum and thinner adipose subcutaneous tissue in children provide ideal acoustic windows.1,2
There are limitations to plain chest radiography, such as poor image quality, the presence of artefacts, the time required to obtain the image and the exposure to ionising radiation. There are also limitations to chest computed tomography (CT), the gold standard for the diagnosis of respiratory pathology, including its high cost, reduced availability, higher exposure to radiation and difficulty involved in transporting the patient outside the unit.3 Thus, PoCUS is emerging as the ideal diagnostic tool in the paediatric intensive care unit (PICU): quick, non-invasive, repeatable, offering real-time information and without exposure to radiation, with sensitivities and specificities that approximate those of computed tomography (CT).4,5
We present five clinical cases of common diseases in the PICU in which PoCUS was a useful diagnostic tool and guided changes to the therapeutic approach.
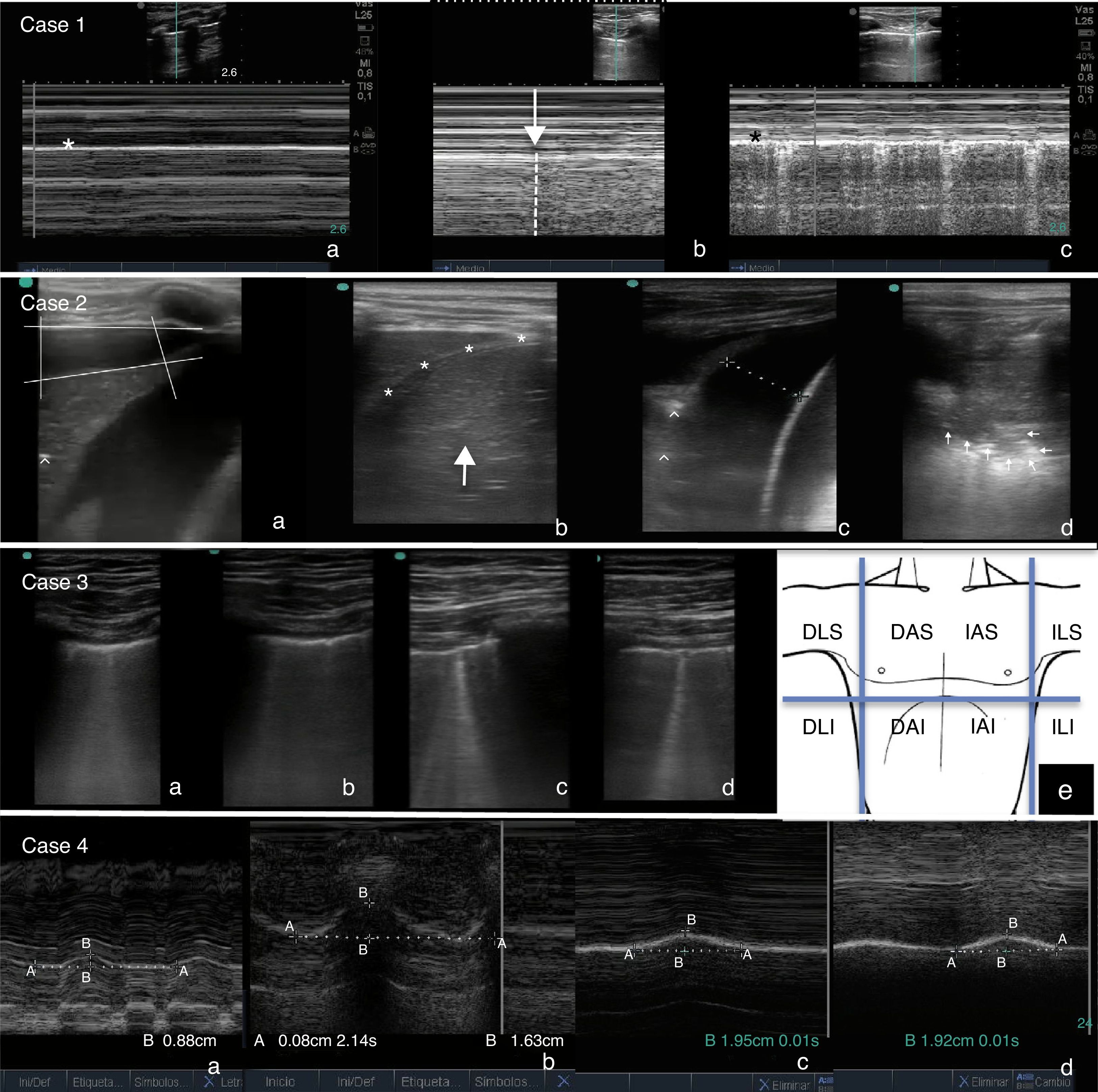
Case 1Infant aged 2 months admitted for bronchiolitis. After being intubated, the patient had difficulty with oxygenation and ventilation, leading to suspicion of a secondary pneumothorax. PoCUS: sonographic signs of right-sided pneumothorax (absence of lung sliding, stratosphere sign, absence of B lines, presence of lung point). After insertion of a pleural drainage tube, the resolution of pneumothorax was detected by ultrasound. The use of PoCUS prevented diagnostic and treatment delays (Fig. 1).
Case 1: (a) M-mode. Stratosphere sign: linear pattern at both sides of the pleural line (*), indicating the absence of lung sliding. (b) M-mode. Lung point: transition from the normal lung (right side, linear granular pattern) to the pneumothorax (left side, linear pattern). (c) M-mode after resolution of pneumothorax. Seashore sign: combination of a linear patter and a granular pattern at either side of the pleural line (*), indicating normal lung sliding. Case 2: (a) right-sided pleural effusion (quad sign) with consolidated underlying lung parenchyma (tissue-like sign) and bronchograms (hyperechoic foci [^]). (b) Resolution of effusion after chest tube placement. Lung consolidation above the diaphragm (*), difficult to differentiate from the liver (arrow). (c) Left hemithorax: basal pleural effusion and consolidated underlying lung (tissue-like sign and bronchograms [^]). (d) Left hemithorax: lung parenchyma consolidation, with detection of tissue-like sign and shred sign (pattern of consolidation with an irregular lower border indicating where the consolidated parenchyma makes contact with the normal lung tissue [arrows]). Case 3: coalescing B lines in (a) anterior and superior (AS) regions of right hemithorax, (b) anterior and inferior (AI) right hemithorax, (c) AS left hemithorax, (d) AI left hemithorax. Diagnosis of bilateral diffuse interstitial syndrome. (e) Ideal areas for ultrasound examination for the diagnosis of diffuse interstitial lung syndrome. AS, AI, lateral superior (LS), lateral inferior (LI), right (D), left (I). Case 4: M-mode (a) Right diaphragm: maximal diaphragmatic excursion between inspiration and expiration of 0.88cm (50% compared to contralateral excursion). (b) Left diaphragm: 1.63cm. (c and d) Left and right diaphragm after resolution of paralysis (1.96 vs 1.92cm).
Male aged 5 years with pleural effusion associated with right lower lobe pneumonia on the fifth day of antibiotic treatment. PoCUS: right-sided pleural effusion (quad sign) and consolidation of the underlying lung parenchyma (tissue-like sign or hepatisation and bronchograms). A pleural drainage tube was placed, and the draining fluid had normal characteristics. These findings led to performance of a contralateral PoCUS, which revealed a small volume of pleural effusion and consolidation of the underlying lung parenchyma. Following the sonographic diagnosis of bilateral pneumonia, testing for atypical pathogens was requested, the results of which were positive for Mycoplasma pneumoniae and adenovirus. PoCUS was useful in guiding the thoracocentesis and the aetiologic diagnosis, and in finding evidence of consolidation that had not been detected by plain radiography (Fig. 1).
Case 3Male aged 17 years with a history of operated tetralogy of Fallot admitted to the unit for respiratory difficulty and cyanosis following resection of a nasal polyp. At admission, the patient presented with mild tachycardia, tachypnoea, cyanosis, intercostal and suprasternal retractions and rales. PoCUS: pattern of coalescing B lines in anterior regions of both lungs (bilateral diffuse interstitial syndrome). In light of these findings, the tachycardia was reassessed, leading to diagnosis of atrial flutter with 2:1 conduction. The use of PoCUS allowed the diagnosis of acute pulmonary oedema secondary to arrhythmia in a patient with a prior history of ventriculotomy and a difficult-to-interpret ECG (Fig. 1).
Case 4Male aged 7 years that had received a diagnosis of severe Ebstein's anomaly and admitted following surgical closure of atrial septal defect, tricuspid valve repair and a bidirectional Glenn procedure. The patient had a complicated postoperative course with extubation failure requiring rescue noninvasive ventilation. PoCUS: reduced movement of right diaphragm by 50% compared to the left (postoperative phrenic nerve palsy). Given the possible need of diaphragmatic plication due to univentricular physiology, PoCUS was performed daily, showing progressive improvement and ultimately resolution. The use of PoCUS allowed the harmless monitorisation of diaphragmatic function and the adoption of a conservative approach (Fig. 1).
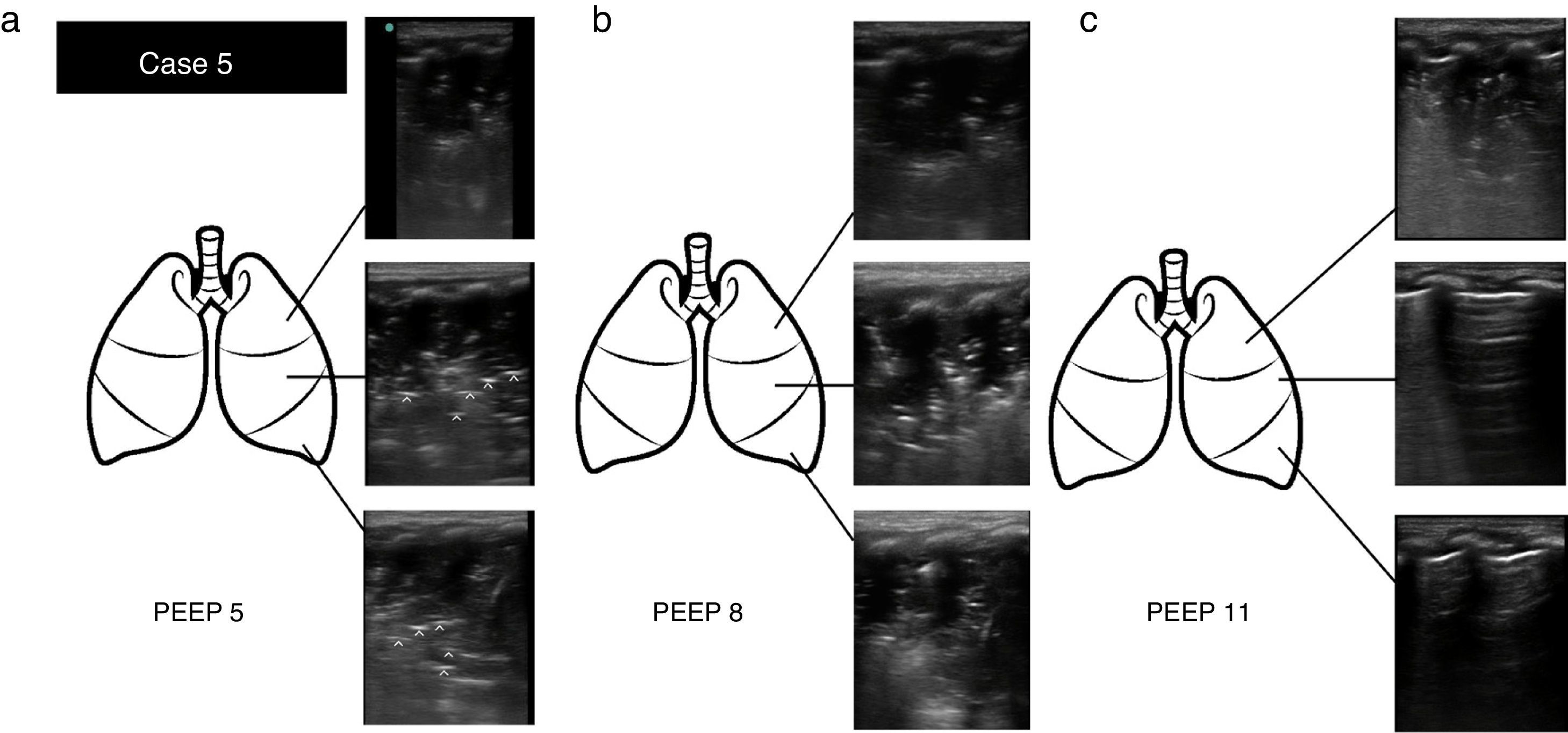
Case 5Infant aged one month admitted for bronchiolitis and on mechanical ventilation. The patient experienced sudden desaturation with a high peak inspiratory pressure (volume-targeted mode of ventilation) and hypoventilation in the left hemithorax. PoCUS: visualisation of consolidation in entire left lung (superior, medial and basal regions), suggestive of atelectasis in the existing clinical context and consistent with the presence of regular parallel bronchograms. Alveolar recruitment measures were initiated, increasing the positive-end expiratory pressure (PEEP) from 5 to 8cm H2O. One hour later the patient had shown clinical but not sonographic improvement. The PEEP was increased again from 8 to 11cm H2O. One hour later, PoCUS revealed a nearly full resolution of the atelectasis, with sonographic signs of a normally aerated lung in the medial and basal regions and consolidation persisting only in the apical region. The use of PoCUS allowed the rapid and differential diagnosis of a severe complication, in addition to guiding the complex alveolar recruitment manoeuvre (Fig. 2).
Case 5: (a) Ultrasound examination at PEEP 5cm H2O. Lung consolidation with detection of tissue-like sign and regular and parallel bronchograms (hyperechoic lines [^]) in the superior, medial and lateral regions. (b) Ultrasound examination at PEEP 8cm H2O. The findings were the same. (c) Ultrasound examination after alveolar recruitment at PEEP 11cm H2O. Evidence of consolidation in the apical region of the lung, with normal aeration in medial and basal lung regions (visualisation of A lines).
The cases presented here demonstrate the usefulness and safety of PoCUS carried out by the paediatric intensivist, with a potentially beneficial impact on the diagnosis and treatment of severely ill children contingent on an appropriate standardised training.5
Please cite this article as: Coca Pérez A, Vázquez Martínez JL, Pérez Caballero Macarrón C, Tapia Moreno R, Stanescu S. Utilidad de la ecografía pulmonar a pie de cama en cuidados intensivos pediátricos. An Pediatr (Barc). 2016;84:57–59.


![Case 1: (a) M-mode. Stratosphere sign: linear pattern at both sides of the pleural line (*), indicating the absence of lung sliding. (b) M-mode. Lung point: transition from the normal lung (right side, linear granular pattern) to the pneumothorax (left side, linear pattern). (c) M-mode after resolution of pneumothorax. Seashore sign: combination of a linear patter and a granular pattern at either side of the pleural line (*), indicating normal lung sliding. Case 2: (a) right-sided pleural effusion (quad sign) with consolidated underlying lung parenchyma (tissue-like sign) and bronchograms (hyperechoic foci [^]). (b) Resolution of effusion after chest tube placement. Lung consolidation above the diaphragm (*), difficult to differentiate from the liver (arrow). (c) Left hemithorax: basal pleural effusion and consolidated underlying lung (tissue-like sign and bronchograms [^]). (d) Left hemithorax: lung parenchyma consolidation, with detection of tissue-like sign and shred sign (pattern of consolidation with an irregular lower border indicating where the consolidated parenchyma makes contact with the normal lung tissue [arrows]). Case 3: coalescing B lines in (a) anterior and superior (AS) regions of right hemithorax, (b) anterior and inferior (AI) right hemithorax, (c) AS left hemithorax, (d) AI left hemithorax. Diagnosis of bilateral diffuse interstitial syndrome. (e) Ideal areas for ultrasound examination for the diagnosis of diffuse interstitial lung syndrome. AS, AI, lateral superior (LS), lateral inferior (LI), right (D), left (I). Case 4: M-mode (a) Right diaphragm: maximal diaphragmatic excursion between inspiration and expiration of 0.88cm (50% compared to contralateral excursion). (b) Left diaphragm: 1.63cm. (c and d) Left and right diaphragm after resolution of paralysis (1.96 vs 1.92cm). Case 1: (a) M-mode. Stratosphere sign: linear pattern at both sides of the pleural line (*), indicating the absence of lung sliding. (b) M-mode. Lung point: transition from the normal lung (right side, linear granular pattern) to the pneumothorax (left side, linear pattern). (c) M-mode after resolution of pneumothorax. Seashore sign: combination of a linear patter and a granular pattern at either side of the pleural line (*), indicating normal lung sliding. Case 2: (a) right-sided pleural effusion (quad sign) with consolidated underlying lung parenchyma (tissue-like sign) and bronchograms (hyperechoic foci [^]). (b) Resolution of effusion after chest tube placement. Lung consolidation above the diaphragm (*), difficult to differentiate from the liver (arrow). (c) Left hemithorax: basal pleural effusion and consolidated underlying lung (tissue-like sign and bronchograms [^]). (d) Left hemithorax: lung parenchyma consolidation, with detection of tissue-like sign and shred sign (pattern of consolidation with an irregular lower border indicating where the consolidated parenchyma makes contact with the normal lung tissue [arrows]). Case 3: coalescing B lines in (a) anterior and superior (AS) regions of right hemithorax, (b) anterior and inferior (AI) right hemithorax, (c) AS left hemithorax, (d) AI left hemithorax. Diagnosis of bilateral diffuse interstitial syndrome. (e) Ideal areas for ultrasound examination for the diagnosis of diffuse interstitial lung syndrome. AS, AI, lateral superior (LS), lateral inferior (LI), right (D), left (I). Case 4: M-mode (a) Right diaphragm: maximal diaphragmatic excursion between inspiration and expiration of 0.88cm (50% compared to contralateral excursion). (b) Left diaphragm: 1.63cm. (c and d) Left and right diaphragm after resolution of paralysis (1.96 vs 1.92cm).](https://static.elsevier.es/multimedia/23412879/0000008400000001/v3_201703220337/S2341287915002859/v3_201703220337/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95erwEulN6Tmh1xJpRhO+VE=)
![Case 5: (a) Ultrasound examination at PEEP 5cm H2O. Lung consolidation with detection of tissue-like sign and regular and parallel bronchograms (hyperechoic lines [^]) in the superior, medial and lateral regions. (b) Ultrasound examination at PEEP 8cm H2O. The findings were the same. (c) Ultrasound examination after alveolar recruitment at PEEP 11cm H2O. Evidence of consolidation in the apical region of the lung, with normal aeration in medial and basal lung regions (visualisation of A lines). Case 5: (a) Ultrasound examination at PEEP 5cm H2O. Lung consolidation with detection of tissue-like sign and regular and parallel bronchograms (hyperechoic lines [^]) in the superior, medial and lateral regions. (b) Ultrasound examination at PEEP 8cm H2O. The findings were the same. (c) Ultrasound examination after alveolar recruitment at PEEP 11cm H2O. Evidence of consolidation in the apical region of the lung, with normal aeration in medial and basal lung regions (visualisation of A lines).](https://static.elsevier.es/multimedia/23412879/0000008400000001/v3_201703220337/S2341287915002859/v3_201703220337/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95erwEulN6Tmh1xJpRhO+VE=)

