The screening program or early detection of congenital hypothyroidism is one of the greatest advances achieved in Pediatrics. Thyroid hormones are essential for brain development and maturation, which continue into the neonatal stage. Alterations in thyroid function in premature and underweight children in the first months of life causes irreversible damage to the central nervous system and is one of the most frequent and avoidable causes of mental retardation. Diagnosis in the neonatal period is difficult, so it requires an analytical study to be able to carry out the appropriate treatment.
The relevance of this problem justifies its communication to all areas of pediatrics. The main objective is to avoid brain damage in these patients. Other aspects to optimize the adequate development of these children with all the necessary periodic controls and to achieve the inclusion of the diagnosis of thyroid alterations during the stay in neonatal units and in the first months of life, need to implement the resources of the health centers and continue advancing according to current knowledge.
In this document, we will focus on the screening of preterm newborns VLBW (<32 weeks of gestation) and/or very low weight for gestational age (1500–1000 g VLBW or <1000 g) and the function evaluation protocol thyroid in premature babies.
We update the diagnostic procedures, the essential and complementary tests required, the etiology and the differential diagnoses in this pathology.
El Programa de cribado o detección precoz del hipotiroidismo congénito es uno de los mayores avances logrados en Pediatría. Las hormonas tiroideas son imprescindibles para el desarrollo y la maduración cerebral, que continúan en la etapa neonatal. Las alteraciones de la función tiroidea en niños prematuros y con bajo peso en los primeros meses de vida origina lesiones irreversibles en el sistema nervioso central y es una de las causas más frecuentes y evitables de retraso mental. El diagnóstico en el periodo neonatal es difícil, por lo que requiere estudio analítico para poder efectuar el tratamiento adecuado.
La relevancia de este problema justifica su difusión a todas las áreas de Pediatría. El objetivo principal, evitar el daño cerebral en estos pacientes. Otros aspectos para optimizar el desarrollo adecuado de estos niños con todos los controles periódicos necesarios y lograr la inclusión del diagnóstico de las alteraciones tiroideas durante la estancia en unidades neonatales y en los primeros meses de vida precisan implementar los recursos de los centros sanitarios y continuar avanzando según los conocimientos actuales.
En el presente documento nos centraremos en el cribado de los recién nacidos pretérmino (<32 semanas de gestación) o con muy bajo peso para la edad gestacional (1.500–1.000 g muy bajo peso al nacer, o <1.000 g peso extremadamente bajo al nacer) y la protocolización de evaluación de función tiroidea en prematuros.
Actualizamos los procedimientos diagnósticos, las pruebas imprescindibles y complementarias requeridas, la etiología y los diagnósticos diferenciales en esta patología.
The incidence of congenital hypothyroidism (CH) has increased globally and is at approximately 1 case per 1400–1700 live births.1 The reasons for this increase include a decrease in the cut-off values of thyroid-stimulating hormone (TSH) applied in newborn screening,2 the increased survival of preterm (PT) babies2 and the implementation of strategies for detection of CT in PT newborn infants.
Congenital hypothyroidism is one of the preventable causes of intellectual disability. Newborn screening of CH was developed to prevent such neurodevelopmental sequelae. Different methods are used for screening; most newborn screening protocols in Spain include measurement of TSH in a dried blood spot sample, others call for measuring total thyroxine (T4) levels in a dried blood spot sample and measurement of TSH levels based on the results, and others call for initial measurement of both TSH and T4.
In this article, we focus on the newborn screening for PT infants (born at gestational age [GA] ≤32 weeks) or small for gestational age (very low birth weight [VLBW], birth weight of 1500–1000 g or extremely low birth weight [ELBW], birth weight less than 1000 g) and the protocol for evaluation of thyroid function.
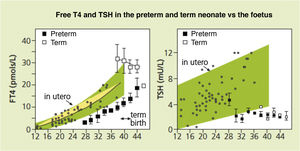
Foetal thyroid physiologyThe thyroid gland develops throughout the foetal period3,4 (Fig. 1).
Serum concentrations of free thyroxine (FT4) in preterm infants born at 27–36 weeks of gestation at different time points post birth are not only lower compared to term infants of the same postmenstrual age, but also clearly lower than the concentrations that would have been achieved in utero.
In the first trimester, the T4 circulating in the baby comes from the mother, as the baby does not produce a sufficient amount of T4 until the second half of gestation. From that point, there is an increase in the concentration of T4 due to liver production of thyroxine-binding globulin (TBG) and production of T4 by the foetal thyroid gland stimulated by TSH. The concentration of T4 increases from 2 µg/dL at 12 weeks to 10 µg/dL in term neonates, the concentration of free thyroxine (FT4) from 0.1 ng/dL at 12 weeks to 2 ng/dL at term, and triiodothyronine (T3) and free triiodothyronine (FT3) concentrations do not increase in the foetus due to placental deiodinase activity except at the end of gestation5 (Table 1).
Thyroid development during gestation.
| Desarrollo fetal | Weeks of gestation |
|---|---|
| The thyroid gland is the first endocrine gland to develop | 7 |
| Hypothalamus (TRH secretion) | 6−8 |
| Capacity to concentrate iodine in vivo (coinciding with the development of the thyroid follicles and colloid) | 10−12 |
| Hypophyseal portal system | 8−12 |
| Pituitary gland starts to develop (TSH secretion) | 12 |
| Start of hypothalamic-pituitary-thyroid axis | 20 |
| Start of T4 secretion | From 22−24 |
| Start of T3 secretion | From 26−28 |
| Deiodinase enzyme system | From 30 |
In addition, the iodine that the foetus needs to synthesise hormones comes from the mother and is transferred through the placenta, with foetal requirements amounting to 250–300 µg/day.6
The thyroid gland is smaller in preterm infants, which results in reduced production of thyroid hormones and a decreased capacity for iodine storage, leading to impaired thyroid function at a time when hormone requirements change rapidly.
Thyroid function in term infantsIn the 30–60 min post birth, due to the colder environment and umbilical cord clamping, the production of TSH by the infant increases to 60–80 mIU/L to then decrease to 20 mIU/L at 24 h and post birth and 6–8 mIU/L by approximately 1 week post birth.7 There is also an increase in T4 and FT4 levels (to 10–22 µg/dL and 2–5 ng/dL, respectively) at 24–36 h post birth. Levels of T3 also increase due to the increased secretion and conversion of T4 to T3 in tissues.
The levels of T4, FT4 and T3 decrease gradually in the first 4 weeks post births to 7–16 µg/dL of T4, 0.8–2 ng/dL of FT4 and 0.5–6 mIU/L of TSH.
Thyroid function in preterm infantsFollowing birth, PT infants also experience a similar increase in TSH and thyroid hormone levels, but of lesser magnitude compared to term infants. The levels are proportional to the weeks of gestation and birth weight5,8,9 (Fig. 1).
Murphy et al.8 analysed the changes in the hypothalamic-pituitary-thyroid (HPT) axis in the first 24 h post birth in PT infants born at 24 and 34 weeks of GA and compared them based on GA. They found that the TSH peak that occurs around birth was attenuated in the group born at 24–27 weeks; the levels of T4 also declined in the first 24 h in this group, while it increased in the more mature group. As occurs in term infants, T4 values decrease in the first week of life, but this decrease is greater in PT and VLBW infants, as clearance of T4 is quicker. There are several possible causes of impaired thyroid function in PT infants, which we present in Table 2.
Causes of impaired thyroid function in preterm infants.
| Loss of transfer of maternal T4 through the placenta |
| Immaturity of HPT axis |
| Limited reserves in thyroid gland due to small size |
| Persistence of foetal thyroid hormone metabolism |
| Predisposition to nonthyroidal illness?: perinatal medication and adverse events. Proposal: changes due to medication and severe perinatal disease |
| Iodine lack, deficiency or excess? |
Normal values in PT infants relative to GA and postnatal age have yet to be established,10 which may result in overdiagnosis or underdiagnosis of hypothyroidism in these patients.
Carrascosa et al.11 analysed thyroid function in 75 healthy PT infants born between 30 and 35 weeks of gestation through the first year of life in comparison to term infants of the same postnatal age. The mean TSH concentration at 24 h post birth in PT infants was significantly lower. Also, the levels of T4 and T3 at 1 and 24 h were significantly lower and the level of reverse triiodothyronine (rT3) at 24 h significantly higher in PT infants. From 1 week post birth, the values obtained in thyroid function tests were in the same range in both groups.
Thyroid function may be abnormal in PT infants with hyaline membrane disease or respiratory distress, manifesting as euthyroid sick syndrome.12 The expected increase in TSH, T4 and T3 concentrations at birth does not occur, and levels may not increase until the child recovers and will do at a very slow pace. There is also an inverse correlation between FT4 levels and disease severity in these infants.
Infants born small for gestational age also exhibit particular alterations of thyroid function. Their TSH levels increase at birth, but stay within the normal range, and they have greater thyroid hormone requirements in the long term, similar to preterm infants, and therefore should be monitored at regular intervals.13
In short, the maturation of the HPT axis in PT and SGA newborns with an increase in TSH levels occurs between 2 and 6 weeks post birth. Despite these differences and the presence of factors that affect thyroid function in premature infants, the cut-off values used for diagnosis of CH are the same that are applied in term infants, which increases the probability of false negatives in screening, as noted above.
Specific issues in very or extremely low birth weight infantsThese patients frequently exhibit thyroid function abnormalities, such as delayed thyrotropin elevation, transient hypothyroxinaemia or hypothyroxinaemia. These abnormalities may be transient or persistent.
In recent years, there has been evidence of an increased incidence of CH in PT infants1 that could exceed the incidence found in term neonates, reaching up to 1 case per 400 preterm births. It is unclear whether this increase is real or is due to more frequent detection in PT infants of moderate or transient forms of CH.
Delayed thyrotropin elevationPreterm infants may experience delayed TSH elevation, in which case newborn screening would not detect the problem. There is evidence that the results of newborn screening may be normal in 5%–10% of VLBW or ELBW infants.14
Delayed TSH elevation in PT neonates is not well understood, and whether it is a transient disorder due to the immaturity of the axis or constitutes a moderate but persistent form of CH continues to be debated.
There are international recommendations for the detection of CH in these cases, among which we ought to highlight the following:
- –
In the document published jointly by the American Academy of Pediatrics, American Thyroid Association and Lawson Wilkins Pediatric Endocrine Society in 2006,15 the authors acknowledged that delayed TSH elevation is more frequent in preterm infants but also discussed the difficulties of implementing a universal screening programme with routine testing of a second specimen and expressed the need of longitudinal studies to assess the long-term outcomes of these measures. An alternative option would be to limit this screening approach to patients at high risk of CH, such as VLBW infants (incidence of CH, 1 case per 250 births), ELBW infants (incidence of CH, 1 case per 1589 births) or newborn infants admitted to the neonatal intensive care unit or with cardiovascular disease, with re-evaluations at 2 and 6 weeks. In case of persistent hyperthyrotropinaemia at 6 weeks, the guideline suggests initiation of treatment and retesting at 3 years.
- –
The consensus guidelines of the European Society of Paediatric Endocrinology published in 201416 recommended a second-screening approach in the following cases: preterm neonates with a GA of less than 37 weeks; LBW and VLBW neonates; ill and preterm neonates admitted to the neonatal intensive care unit; specimen collection within the first 24 h of life; and multiple births, particularly in cases of same-sex twins. They recommended collection of the second specimen at 2 weeks post birth or 2 weeks after the initial screening. Still, the authors recognised that this approach is not implemented in every neonatal unit and underscored the difficulty of implementing the recommendation of retesting with venous blood specimens instead of dried blood spot specimens.
Data have been obtained through the implementation of specific programmes for rescreening of thyroid function in PT infants.17 The observed frequency of CH was higher in PT infants compared to term infants, with an incidence of 1 per 579 PT births at 32–36 weeks of gestation versus 1 per 1488 term births at 37 or more weeks of gestation. The incidence was higher in infants born at or before 32 weeks: 1.56% in low birth weight infants, 1.9% in VLBW infants and 3.7% in ELBW infants.
McGrath et al.18 collected whole blood samples weekly through 37 weeks of corrected age or hospital discharge. The authors highlighted that 27 (50.9%) PT infants born before 33 weeks of gestation that received a diagnosis of CH had delayed TSH elevation detected between 8 and 48 days post birth (mean, 13 days) that would not have been detectable in the initial screen. Of these patients, 12 (40.7%) had decompensated hypothyroidism (FT4 < 10 pmol/L) and 4 had severe hypothyroidism (FT4 < 5.5 pmol/L). This study also analysed repeat screening, which identified 6 cases (22%) of permanent CH and 8 (29%) of transient CH. In 13 neonates (48%) elevation of TSH was detected after 15 days, and 7 of them had a FT4 concentration below 10 pmol/L. In addition, 25% of infants with delayed TSH elevation had a history of iodine exposure, so the authors recommended monitoring for up to 1 month after exposure.
Kaluarachchi et al.19 conducted screening in heel prick blood samples at 2 weeks, 4 weeks and discharge, with testing of venous samples performed for confirmation. They diagnosed CH in 49 PT infants, of who 92% had delayed TSH elevation. Testing of samples obtained at 2 weeks identified the most cases (n = 18), and it is worth highlighting that 15 patients had TSH concentrations greater than 100 mU/L and that 1 patient with early TSH elevation and 19 with delayed TSH elevation had free T4 concentrations of less than 0.8 ng/dL, which seems to justify monitoring with retesting at different time points.
In short, given the high incidence of abnormalities in these patients and the potential impact on their outcomes, it seems reasonable to recommend reassessment of thyroid function and monitor the patient, as the long-term impact of diagnosis or treatment in these cases is unknown.
Transient elevation of TSHTransient hyperthyrotropinaemia can be due to different factors: maternal thyroid disease (maternal treatment with antithyroid agents, transfer of maternal TSH receptor antibodies [TRAB]), gene variants (heterozygous variants in DUOX-2 gene or the TSHR gene that encodes the TSH receptor), prenatal/postnatal exposure to high doses of iodine (povidone-iodine, iodinated contrast media), areas of natural iodine deficiency, factors related to disease severity or use of drugs listed in Table 3.
Drugs that affect thyroid function.
| Decrease or increase thyroid hormone secretion | Dopamine glucocorticoids octreotide |
|---|---|
| Decrease TSH secretion | Iodine |
| Amiodarone | |
| Increase the concentration of TBG | Oestrogens |
| Decrease the concentration of TBG | Glucocorticoids |
| Protein-binding site displacement | Furosemide |
| Salicylate | |
| Increase liver metabolism | Phenobarbital |
| Phenytoin | |
| Carbamazepine | |
| Decrease T4 5’-deiodinase activity | Propylthiouracil |
| Amiodarone | |
| Beta-blockers | |
| Glucocorticoids |
TBG, thyroxin-binding globulin; TSH, thyroid-stimulating hormone.
In recent years, improvements in treatment and the management of perinatal disease (antenatal steroid therapy, noninvasive ventilation, decreased used of pharmaceuticals etc.) has achieved a decrease in the incidence of hypothyroxinaemia of prematurity and delayed hyperthyrotropinaemia in extremely PT infants.20
Persistent elevation past 2 weeks post birth with a TSH concentration greater than 10 mU/L or a FT4 concentration of less than 0.8 ng/dL is an indication for treatment recognised in most guidelines. There is less consensus as regards the approach to intermediate TSH values between 6 and 10 mU/L, which will depend on several factors, and in these cases decision-making is shared by the clinician and the parents. Based on the consensus guidelines of the European Thyroid Association, infants with persistent TSH concentrations greater than 10 mU/L after 1 month post birth are eligible for treatment through age 3 years with subsequent reassessment.20 Thyroid imaging (ultrasound or 131I o 99Tc scintigraphy) is recommended to assess for the presence of structural anomalies that would support a diagnosis of permanent CH. The identification of genetic changes associated with hyperthyrotropinaemia can also guide the decision to treat and predict the course of disease.
Hypothyroxinaemia in preterm infantsPreterm neonates are more likely to develop hypothyroxinaemia (low T4/FT4, normal TSH), which is detected in the first weeks of life in up to 50% of those born before 28 weeks of GA and more marked the more immature or severely ill the infant is (Table 4).21
Neonatal conditions associated with abnormal thyroxine concentrations.
| Acute respiratory distress syndrome |
| Bronchopulmonary dysplasia |
| Early and late-onset sepsis |
| Meconium aspiration syndrome |
| Pneumothorax |
| Asphyxia |
| Persistent pulmonary hypertension |
| Necrotising enterocolitis |
| Patent ductus arteriosus |
Most cases are transient, but in the early stages it may be challenging for the clinician to discern whether the hypothyroxinaemia represents a secondary or tertiary form of hypothyroidism (hypothalamic-pituitary) or is due to TBG deficiency. The current scientific evidence is also insufficient to prove that levothyroxine for treatment of hypothyroxinaemia in PT infants can improve long-term neurodevelopmental outcomes. There are few randomised trials, conducted in small samples (10–100 preterm infants), with heterogeneity in the characteristics of participants and the protocols used (doses of levothyroxine ranging from 4 to 20 µg/kg/day, duration ranging from 2 to 6 weeks), which hinders interpretation of the data.22 A recent review23 analysed 20 articles on the impact of hypothyroxinaemia on neurodevelopment and 7 randomised trials on hormone replacement therapy with levothyroxine conducted between 1981 and 2016. Some of the evidence suggests that hormone replacement could be beneficial for extremely PT infants. Other studies have found an association between T4/T3 levels and patient outcomes (mortality, cardiovascular complications, etc.), although it would be difficult to establish causality.24,25
Treatment with thyroxine remains controversial,26–30 and the current evidence does not support recommending its routine use of treatment of PT infants with transient hypothyroxinaemia. More data is required to identify cases in which treatment may be beneficial,30 the best time to initiate treatment and its ideal duration based on the optimal cut-off points for circulating thyroid hormone levels.
We would recommend initiation of treatment in case of hypothyroxinaemia associated with an elevated TSH concentration greater than 10 mU/L or persistent elevation (FT4 < 0.8 ng/dL in 2 measurements taken 1–2 weeks apart) with the decision to treat made on a case-by-case basis in patients at high risk, such as PT infants born before 28 weeks’ gestation or with weights of less than 1000 g, especially those with severe illness (Table 5).
Recommendation for treatment of hypothyroxinaemia in preterm infants.
| The current evidence does not support recommendation of routine treatment in PT infants with transient hypothyroxinaemia. |
| We would recommend initiation of treatment in case of hypothyroxinaemia associated with elevation of TSH, and individualized treatment in patients with risk factors such as severe illness |
It is important to differentiate transient hypothyroxinaemia of prematurity from hypothyroxinaemia associated with abnormalities in the hypothalamic-pituitary axis. Persistent low FT4 levels combined with levels of TSH below or in the lower range of normal is indicative of central CH. It is most frequently associated with other pituitary hormone deficiencies (congenital panhypopituitarism) and may manifest with prolonged neonatal hypoglycaemia, micropenis or bilateral cryptorchidism and prolonged jaundice, in addition to abnormal pituitary gland morphology (ectopic neurohypophysis, anterior pituitary agenesis or hypoplasia, pituitary stalk anomalies, or midline anomalies such as septo-optic dysplasia). There is also the possibility of isolated central hypothyroidism, which is infrequent, with an incidence of 1 per 30 000 births, and cannot be detected by newborn screening of TSH levels.
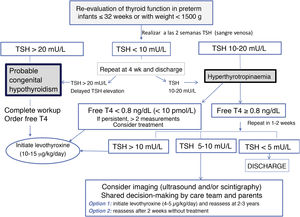
Standardization of thyroid function assessment in preterm infantsHaving analysed the particular characteristics of thyroid function in PT infants, the need to screen for hypothyroidism differently than in term infants becomes apparent (Fig. 2).
Therefore, the protocol that we recommend for detection of thyroid dysfunction in PT neonates born at or before 32 weeks’ gestation or with weights of less than 1500 is as follows31–33:
- 1
We recommend measurement of thyroid hormone levels (TSH/FT4) in venous blood specimens at 2 weeks, 4 weeks, on reaching a weight of 1500 g or at discharge, or, in autonomous communities that include these measurements in the newborn screening programme, measurement of TSH in dry blood spot specimens can also be considered (Table 6).
Table 6.Recommendations for reassessment of thyroid function in preterm infants born at or before 32 weeks’ gestation or with weights of less than 1500 g.
We recommend evaluation in every infant born at GA ≤ 32 weeks or with a birth weight <1500 g Assessment at 2 weeks post birth or 2 weeks from the initial screen, to be repeated at 4 and 10 weeks and then at discharge or when the infant reaches a weight of 1500 g.32,33 In addition, we recommend retesting in infants with weights ≥ 1500 g if they continue to be critically ill. Ideally, screening will consist of measurement of TSH and FT4 in venous blood, or measurement of TSH in a dried blood spot in centres where this technique is available. The decision to monitor or treat will be made following the proposed algorithm (Fig. 2)
The risk factors for CH should be assessed, and therefore it is important to inquire about the use of medication that could interfere with thyroid function, the use of povidone-iodine or iodinated contrast.
- 2
Patients with abnormal test results at 2 weeks:
- none–
Require treatment if the venous TSH concentration is below 20 mU/L. The workup is completed with testing of free T4, but treatment initiation is not delayed, and the thyroid should be imaged using scintigraphy (with or without the perchlorate discharge test), ultrasonography or both.
- none–
Require re-evaluation in case of a venous TSH concentration between 10 and 20 mU/L. Since some cases are transient, retesting is recommended at 1–2 weeks, contemplating treatment if the abnormal results persist.
- none–
If abnormal results persist, with a TSH concentration of 5–10 mU/L at 3–4 weeks post birth or before discharge, we recommend expanding the workup (thyroid imaging, serum thyroglobulin) and considering treatment with levothyroxine, with retesting at age 2 or 3 years or even earlier in patients with levothyroxine requirements of less than 3 µg/kg/day, given that many of these cases are transient34 (Table 7).
Table 7.Recommendations on hyperthyrotropinaemia (elevation of TSH levels with normal FT4 levels).
Elevation of TSH at 2 weeks post birth requires retesting at 2–4 weeks. We recommend treatment if elevation of TSH above 10 mU/L persists (Fig. 2) and retesting in patients with concentrations between 5 and 10 mU/L. In patients with hyperthyrotropinaemia (TSH > 10 mU/L) + hypothyroxinaemia (FT4 < 0.8 ng/dL or <10 pmol/L), we recommend initiation of treatment in every case in addition to retesting If elevation of TSH above 10 mU/L persists after 1 month post birth, we recommend considering replacement with levothyroxine and reassessment at age 2–3 years.21 Earlier reassessment may be considered in PT infants with a confirmed eutopic thyroid gland that required low doses of levothyroxine during follow-up (<3 µg/kg/day), as thyroid function may have normalised in 38%–55% of these patients.24
- none–
- 1
PT infants born before 32 weeks’ gestation or with very low birth weight (<1500 g) are at risk of impaired thyroid function. A normal TSH concentration in the newborn screening performed in the first days post birth does not rule out CH in PT infants.
- 2
Due to the risk of false negatives in the newborn HC screen and the risk of thyroid dysfunction, we recommend repeating the screening at 2 weeks post birth, 4 weeks post birth, on achieving a weight of 1500 g or at discharge.32,33
- 3
Total and free T4 concentrations should normalise after 2–8 weeks in low birth weight infants (born at 28–32 weeks or with birth weights of 1000–1500 g) or 4–12 weeks in ELBW infants (born before 28 weeks or with birth weights <1000 g).
- 4
The current evidence does not support the recommendation of routine treatment of transient hypothyroxinaemia in PT infants. Initiation of treatment is recommended in cases in which hypothyroxinaemia is associated with elevation of TSH past 10 IU/L or persistent elevation of TSH, with individualised treatment in high-risk preterm infants with severe disease.
- 5
A TSH concentration of 20 mU/L or greater combined with any concentration of FT4 is considered as CH and requires treatment at a dose of 10–15 µg/kg/day.
- 6
TSH levels between 10 and 20 mU/L with normal FT4 levels require re-evaluation, with a more thorough investigation by the endocrinologist (including imaging) and contemplation of treatment in case they persist.
The authors have no conflicts of interest to declare.
Please cite this article as: Ares Segura S, Casano-Sancho P, Chueca Guindulain M. Evaluación de la función tiroidea en el recién nacido pretérmino o de muy bajo peso. An Pediatr (Barc). 2021;95:277.