Non-specific acute abdominal pain is the most common process requiring differential diagnosis with appendicitis in clinical practice. The aim of this study was to assess the Paediatric Appendicitis Score in differentiating between these two entities.
Material and methodsAll patients admitted due to suspicion of appendicitis were prospectively evaluated in our hospital over a two-year period. Cases of non-specific acute abdominal pain and appendicitis were enrolled in the study. Several variables were collected, including Score variables and C-reactive protein levels. Descriptive, univariate and multivariate analyses and diagnostic accuracy studies (ROC curves) were performed.
ResultsA total of 275 patients were studied, in which there were 143 cases of non-specific acute abdominal pain and 132 cases of appendicitis. Temperature and right iliac fossa tenderness on palpation were the variables without statistically significant differences, and with no discrimination power between groups. Pain on coughing, hopping, and/or percussion tenderness in the right lower quadrant was the variable with greater association with appendicitis. The Score correctly stratified the patients into risk groups. Substitution of temperature for C-reactive protein in the Score increased diagnostic accuracy, although with no statistically significant differences.
ConclusionsThe Paediatric Appendicitis Score helps in differential diagnosis between appendicitis and non-specific acute abdominal pain. It would be advisable to replace the temperature in the Score, since it has no discrimination power between these groups. C-reactive protein at a cut-off value of 25.5mg/L value could be used instead.
El dolor abdominal agudo inespecífico es el principal proceso que requiere diagnóstico diferencial con la apendicitis en la práctica clínica. El objetivo de este estudio es evaluar la utilidad del Pediatric Appendicitis Score (Regla de predicción clínica de apendicitis pediátrica) para diferenciar estas 2 entidades.
Material y métodosSe evaluó prospectivamente a los pacientes atendidos por sospecha de apendicitis en nuestro centro durante 2 años, incorporando al estudio casos de dolor abdominal agudo inespecífico y apendicitis. Se recogieron diferentes variables, incluyendo las que conforman el Score y la proteína C reactiva, que se analizaron estadísticamente de manera descriptiva, univariante y multivariante, y mediante pruebas de rendimiento diagnóstico (curvas ROC).
ResultadosSe estudiaron 275 casos; 143 casos de dolor abdominal agudo inespecífico y 132 casos de apendicitis. La temperatura y el dolor a palpación en fosa iliaca derecha fueron las únicas variables que no mostraron diferencias significativas entre los grupos, careciendo de poder de discriminación. El dolor con la tos, el salto y/o la percusión fue la variable con mayor asociación a apendicitis. El Score estratificó correctamente a los pacientes en grupos de riesgo. La sustitución de la temperatura por la proteína C reactiva en el Score aumentaba su rendimiento diagnóstico, aunque sin diferencias significativas.
ConclusionesEl Pediatric Appendicitis Score ayuda en el diagnóstico diferencial entre apendicitis y dolor abdominal agudo inespecífico. Sería recomendable la sustitución de la temperatura en el Score, pues carece de poder de discriminación entre estos grupos. La proteína C reactiva, categorizada en el valor 25,5mg/L, podría ser utilizada en su lugar.
Appendicitis accounts for 10% of the cases of abdominal pain assessed in emergency departments and it is the most common acute surgical disease of the abdomen.1 Its diagnosis is mostly based on the findings of the history taking and the physical examination, although other tools, such as measurement of inflammatory markers, imaging tests and clinical prediction rules, may help the process.2 Nevertheless, the rate of misdiagnosis can reach up to 30% due to the low specificity of the symptoms and the broad differential diagnosis of abdominal pain.3
The Paediatric Appendicitis Score (PAS) (Table 1) is the paediatric clinical prediction rule validated most appropriately and stands out in its ability to stratify patients by risk group.4,5 The inflammatory markers included in this prediction rule (total leukocyte and neutrophil counts) and the level of C-reactive protein (CRP) are the most useful markers in the diagnosis of appendicitis.6,7 Since nonspecific abdominal pain (NSAP) is the most frequent diagnosis at discharge from the emergency department in cases of acute abdominal pain, and this is the process that most frequently requires differential diagnosis with appendicitis,8,9 the objective of this study was to assess the usefulness of the PAS in the differential diagnosis of appendicitis and NSAP.
Paediatric Appendicitis Score (clinical prediction rule for paediatric appendicitis).
| Variables | Points |
|---|---|
| Pain in RIF on palpation | 2 |
| Tenderness in RIF with coughing, hopping and/or percussion | 2 |
| Migration of pain toward RIF | 1 |
| Anorexia | 1 |
| Nausea/vomiting | 1 |
| Temperature >37.3°C | 1 |
| Leukocytes >10.0×109/L | 1 |
| Neutrophils >7.5×109/L | 1 |
RIF, right iliac fossa.
We conducted a prospective study of all patients aged less than 15 years assessed in the emergency department of our hospital (Complexo Hospitalario Universitario de Vigo) for suspected appendicitis between 2013 and 2014, selecting cases of appendicitis and NSAP. The inclusion criteria were: clinical suspicion of appendicitis; documentation of the variables included in the PAS (pain in the right iliac fossa [RIF] on palpation, tenderness in the RIF with coughing, hopping and/or percussion; history of pain migration toward the RIF; anorexia; nausea and/or vomiting; body temperature; total leukocyte count and total neutrophil count); serum CRP level; consent given by a parent or legal guardian of the patient to access patient data for the purpose of research. The exclusion criteria were: age less than 5 years (the PAS is difficult to obtain and has not been validated for this age group); abdominal pain lasting more than 72h (differential diagnosis of appendicitis and NSAP is rarely needed in such cases) or less than 6h (diagnostic tests are not usually performed at this point); history of disorder of blood or blood-forming organs, malignancy, liver disease or inflammatory disease, current or diagnosed in the month preceding onset, or antibiotic or anti-inflammatory treatment in the month preceding onset (they could alter the levels of inflammatory markers); interval of more than 12h between collection of blood sample for analysis and appendectomy (for the adequate correlation between inflammatory markers and the type of appendicitis). The diagnosis of NSAP was made by exclusion when disease was not detected, with nonspecific findings of abdominal ultrasound in all cases, and absence of antibiotic treatment in the month following the diagnosis (to avoid including cases of appendicitis or infectious disease that had not been detected and resolved with treatment). The study was approved by the Clinical Research Ethics Committee of Galicia (2013/361).
Variables under studyWe collected data for the following variables: age, sex, time elapsed between onset of abdominal pain and performance of blood tests, PAS variables, serum CRP levels and type of appendicitis (suppurative, gangrenous or perforated). Suppurative appendicitis was defined by the presence of neutrophilic infiltrate in the muscularis propria and gangrenous appendicitis by the presence of necrosis in the appendiceal wall.10 Perforated appendicitis was diagnosed based on the presence of a hole in the appendiceal wall or of a free appendicolith in the peritoneal cavity.11 We considered suppurative appendicitis uncomplicated appendicitis, and gangrenous and perforated appendicitis complicated appendicitis. We followed up cases of NSAP through outpatient visits or by phone in the first month from the diagnosis with the purpose of assessing the exclusion criteria.
All laboratory tests were performed in the haematology emergency laboratory (total leukocyte and neutrophil count) and clinical analysis laboratory (CRP) of our hospital, following routine procedure and always using the same method for each variable.
Statistical analysisWe conducted the statistical analysis with the package SPSS 19.0 for Windows (SPSS Inc; Chicago, IL, USA; 2010). We performed a descriptive analysis of all the variables under study, univariate analyses of quantitative variables with the Student t test and of qualitative variables with the χ2 test, and multivariate logistic regression to detect the variables most strongly correlated with appendicitis. We also assessed the diagnostic yield of different quantitative variables and the PAS by measuring the area under the ROC curve (AUC), identifying the cut-off points with the highest discriminatory power for the diagnosis of appendicitis. Lastly, we developed a modified PAS by adding the CRP level, categorised based on the cut-off point, to the original score. We defined statistical significance as a P-value of less than .05 for all tests.
ResultsDuring the period under study, 764 patients underwent evaluation for suspected appendicitis, and the most frequent diagnoses were NSAP, with 327 cases (42%), and appendicitis, with 273 (36%) (Table 2). The total number of patients included after applying the inclusion and exclusion criteria were 275, of who 143 were in the NSAP group and 132 in the appendicitis group (100 cases of uncomplicated appendicitis and 32 of complicated appendicitis [9 gangrenous and 23 perforated]).
Cases of suspected appendicitis (2013–2014).
| NSAP | 327 (42.8%) |
| Appendicitis | 273 (35.7%) |
| Mesenteric lymphadenitis | 35 (4.5%) |
| Acute gastroenteritis | 24 (3.1%) |
| Urinary tract infection | 17 (2.2%) |
| Ovarian cyst | 14 (1.8%) |
| Respiratory tract infection | 11 (1.4%) |
| Other | 63 (8.2%) |
| Total | 764 (100%) |
NSAP: nonspecific abdominal pain.
Table 3 shows the descriptive analysis of the variables under study. The univariate analysis showed statistically significant differences between the NSAP and appendicitis groups in sex, but not in age or duration of symptoms. We ended up removing the variable of pain in the RIF on palpation from the analysis because it was present in all cases. There were statistically significant differences in the rest of the qualitative variables included in the PAS between the NSAP and the appendicitis groups (Table 3). The analysis of these qualitative variables by type of appendicitis also found statistically significant differences in every instance, except for nausea and/or vomiting between the NSAP and uncomplicated appendicitis groups (P=.830) (Table 4).
Descriptive and univariate analysis of NSAP and appendicitis groups.
| Variable | NSAP | Appendicitis | P |
|---|---|---|---|
| Sex, male/female (ratio) | 60/83 (1:1.4) | 93/39 (2.4:1) | <.001a |
| Age, mean (years)±SD | 10.4±2.5 | 9.9±2.5 | .152b |
| Duration of symptoms (h)±SD | 25.8±16.9 | 24.2±15.9 | .406b |
| Tenderness with coughing, hopping and/or percussion, n (%) | 20 (14.0) | 84 (63.6) | <.001a |
| Migration of pain to RIF, n (%) | 18 (12.6) | 70 (53.0) | <.001a |
| Anorexia, n (%) | 37 (25.9) | 88 (66.7) | <.001a |
| Nausea and/or vomiting, n (%) | 80 (55.9) | 94 (71.2) | .009a |
| Body temperature (°C), mean±SD | 37.2±0.9 | 37.1±0.8 | .871b |
| Leukocytes (×109/L), mean±SD | 10.94±5.26 | 15.47±3.99 | <.001b |
| Neutrophils (×109/L), mean±SD | 7.97±5.25 | 12.51±3.93 | <.001b |
| CRP (mg/L), mean±SD | 15.8±22.0 | 37.1±52.1 | <.001b |
CRP, C-reactive protein; NSAP, nonspecific abdominal pain; RIF, right iliac fossa; SD, standard deviation.
Univariate analysis of variables in the NSAP, UA and CA groups.
| Variable | NSAP vs UA, P | NSAP vs CA, P |
|---|---|---|
| Tenderness with coughing, hopping and/or percussion | <.001a | <.001a |
| Migration of pain to RIF | <.001a | <.001a |
| Anorexia | <.001a | <.001a |
| Nausea and/or vomiting | .83a | .003a |
| Body temperature | .05b | .002b |
| Leucocytes | <.001b | <.001b |
| Neutrophils | <.001b | <.001b |
| C-reactive protein | .335b | <.001b |
CA, complicated appendicitis; NSAP, nonspecific abdominal pain; RIF, right iliac fossa; UA, uncomplicated appendicitis.
Body temperature was the only quantitative variable for which there were no differences between the NSAP and appendicitis groups (P=.871); while differences were found for the rest (total leukocyte count, total neutrophil count, and serum CRP level; P<.001) (Table 3). The analysis of these variables by type of appendicitis revealed significant differences in every instance (P<.05) except in body temperature between the NSAP and uncomplicated appendicitis groups (P=.05) and in CRP levels between the NSAP and uncomplicated appendicitis groups (P=.335) (Table 4).
Following the multivariate regression analysis, the only variables that remained in the model were tenderness with coughing, hopping and/or percussion, migration of pain, anorexia, the neutrophil count and the leukocyte count, although the differences were no longer statistically significant for the last one. The variables that exhibited the strongest association with appendicitis were tenderness with coughing, hopping and/or percussion (odds ratio, 20.0) and migration of pain (odds ratio, 11.4) (Table 5).
Logistic regression analysis.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Tenderness with coughing/hopping/percussion | 20.0 | 7.8–51.3 | <.001 |
| Migration of pain to the RIF | 11.4 | 4.5–28.9 | <.001 |
| Anorexia | 7.5 | 3.2–17.5 | <.001 |
| Neutrophils >7.5×109/L | 7.3 | 1.3–41.2 | .025 |
| Leucocytes > 0.0×109/L | 4.9 | 0.7–31.0 | .093 |
CI, confidence interval; OR, odds ratio; RIF, right iliac fossa.
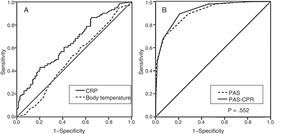
The analysis of the diagnostic yield based on the AUCs of ROC curves for body temperature and inflammatory markers in the appendicitis vs NSAP groups (Table 6) showed that the yield of body temperature was all but negligible (AUC, 0.51), the yield of CRP was low (AUC, 0.64) (Fig. 1) and the yield of leukocyte and neutrophil counts was moderate (AUC, 0.79). In this analysis, the PAS exhibited a high yield (AUC, 0.90), with 3 and 7 being the best cut-off points for the differentiation of low-,1–3 intermediate-4–7 and high-risk groups.8–10 The analysis of the modified PAS where body temperature was replaced by CRP category based on the cut-off point (CRP<25.5mg/L: 0 points; CRP>25.5mg/L: 1 point) showed that it had a higher diagnostic yield compared to the PAS (ROC, 0.92), although the difference was not statistically significant (P=.552) (Table 6) (Fig. 1). The modified PAS improved patient stratification, placing a greater number of NSAP cases in the low-risk group and of complicated appendicitis cases in the high-risk group (Table 7).
Area under ROC curve and cut-off points for variables and the PAS in the differentiation of appendicitis and NSAP.
| Variable | Appendicitis vs NSAP | ||||
|---|---|---|---|---|---|
| AUC | 95% CI | COP | Sen, % | Spe, % | |
| Body temperature (°C) | 0.51 | 0.44–0.57 | 37.2 | 50.8 | 53.1 |
| Leucocytes (×109/L) | 0.79 | 0.74–0.85 | 10.5 | 89.4 | 57.3 |
| Neutrophils (×109/L) | 0.79 | 0.73–0.84 | 7.5 | 90.2 | 60.1 |
| CRP (mg/L) | 0.64 | 0.58–0.71 | 25.5 | 43.2 | 79.7 |
| PAS (1–10) | 0.90 | 0.87–0.94 | 3 | 99.6 | 21.3 |
| 7 | 58.7 | 91.6 | |||
| 3 | 99.6 | 23.1 | |||
| PAS-CRP (1–10) | 0.92 | 0.89–0.95 | 7 | 57.5 | 96.2 |
AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; COP, cut-off point with highest discriminatory power; NSAP: nonspecific abdominal pain; PAS, Paediatric Appendicitis Score; PAS-CRP, modified PAS including CRP; Sen, sensitivity; Spe, specificity.
PAS and modified PAS in NSAP and different types of appendicitis.
| NSAP | UA | CA | |
|---|---|---|---|
| PAS | |||
| 1–3 | 41 (28.7%) | 1 (1.0%) | 0 (0.0%) |
| 4–7 | 101 (70.6%) | 61 (61.0%) | 7 (21.9%) |
| 8–10 | 1 (0.7%) | 38 (38.0%) | 25 (78.1%) |
| PAS-CRP | |||
| 1–3 | 46 (32.2%) | 1 (1.0%) | 0 (0.0%) |
| 4–7 | 95 (66.4%) | 64 (64.0%) | 4 (12.5%) |
| 8–10 | 2 (1.4%) | 35 (35.0%) | 28 (87.5%) |
CA, complicated appendicitis; NSAP: nonspecific abdominal pain; PAS: Paediatric Appendicitis Score; PAS-CRP, Paediatric Appendicitis Score modified to include C-reactive protein; UC, uncomplicated appendicitis.
Nonspecific abdominal pain refers to an acute abdominal pain process without a suspected organic cause that is self-limiting and does not recur. It is a diagnosis of exclusion that is safe in children, as a specific disease is subsequently found in only 1.6% to 5.8% of diagnosed cases with a low associated risk of undetected appendicitis. It is the most frequent discharge diagnosis in cases of acute abdominal pain in paediatric emergency departments, and the process that most frequently requires a differential diagnosis with appendicitis in clinical practice.8,9 In our study, NSAP was the most frequent diagnosis (42%) in cases of suspected appendicitis.
Non-specific abdominal pain is distributed fairly evenly between the two sexes,12 but appendicitis is more frequent in males, with a male: female ratio of 1.5–1.9:1,13 a fact reflected in the larger proportion of this sex in our group of patients (2.4:1 ratio).
Up to 40% to 50% of appendicitis cases may present without one or more of the classic signs or symptoms, such as migration of abdominal pain toward the RIF, signs of peritoneal irritation or anorexia.14 In our study, we found statistically significant differences between cases of appendicitis and cases of NSAP in all the signs and symptoms under study except for body temperature and pain in RIF on palpation, although a considerable percentage of patients did not exhibit a classic presentation.
There is evidence that peritoneal irritation, such as abdominal pain on percussion, and a history of migration of abdominal pain toward the RIF are the strongest predictors of appendicitis.15 In our multivariate analysis, these two variables exhibited the strongest associations with appendicitis, with odds ratios of 20 and 11, respectively. The strong association of coughing, hopping and/or percussion tenderness, which assesses the presence of peritoneal irritation in the PAS, explains the higher weight it is given in the score.
Some studies have already suggested that body temperature is of limited use in the diagnosis of appendicitis.16,17 In our study, body temperature was not useful in differentiating between appendicitis and NSAP.
The inflammatory markers used most commonly in the diagnosis of appendicitis are the total leukocyte count, the total neutrophil count and the serum level of CRP, and to date no other markers have been found that offer better results.6,7 There is considerable variability in the sensitivity and specificity of leukocyte and neutrophil counts reported in the literature, with values ranging from 55% to 89% and from 43% to 66%, respectively.18,19 Their low specificity is due to the elevation of these counts in many other processes that manifest with pain in the RIF.16,20 They are more useful in the first 24h from onset, as inflammatory and infectious processes are associated to neutrophil activation in the first 3–6h from onset.21 For the purpose of diagnosing appendicitis, leukocytes and neutrophils are considered to be elevated when their total counts exceed 10.0×109/L and 7.5×109/L, respectively.4,16,22 The cut-off points with the most discriminatory power and the sensitivity and specificity found in our study were consistent with the existing literature.
There is also considerable variability in the values reported in the literature for the sensitivity and specificity of CRP in the diagnosis of appendicitis, which range from 58% to 100% and from 28% to 93%, respectively.19,23 C-reactive protein is a nonspecific biomarker of inflammation whose synthesis starts 4–6h after the stimulus, with its concentration doubling every 8h, so that serum levels are significantly elevated starting at 12–24h.24 The discriminatory power of CRP for the diagnosis of appendicitis is not high, especially in the early stages, where its value is within ranges compatible with other processes, but CRP levels are useful for differentiating between uncomplicated and complicated appendicitis.19,22,25 Several studies have shown that CRP concentrations between 10 and 50mg/L are associated with uncomplicated appendicitis, with a greater probability of complications (gangrene or perforation) at higher concentrations.6,18,26 In our study, CRP was moderately useful for differentiating between appendicitis and NSAP. The assessment of its diagnostic yield through the analysis of ROC curves identified a cut-off point for discriminating appendicitis that was similar to those reported in the literature (25.5mg/L).
There is evidence that the combination of different inflammatory markers increases their discriminatory and predictive power in the diagnosis of appendicitis.15,17,27 The combination of the total leukocyte count and the CRP level reaches sensitivity and specificity values of 90%–95% for the prediction of appendicitis, with a stronger correlation between elevation of both values and the severity of disease.23,25,26 This increase in discriminatory and predictive power obtained through the combination of the leukocyte count and CRP level makes the addition of the CRP concentration to the PAS variables particularly attractive.
In recent decades, several clinical prediction rules have been developed for the diagnosis of appendicitis in children, and their use in clinical practice has been associated with an increase in diagnostic accuracy and a decreased rate of perforation.28 The PAS prediction rule, published in 2000, is the rule that has been most thoroughly evaluated in the paediatric age group.29–31 It was developed through a multiple linear logistic regression analysis of clinical and laboratory parameters after the prospective evaluation of 1170 children with suspected appendicitis aged 4–15 years. It comprises 8 variables with a statistically significant association that were assigned values based on their sensitivity, specificity, predictive values and diagnostic accuracy, so that the rule categorises patients by risk of appendicitis on a 10-point scale.4 Recent systematic reviews of different appendicitis prediction rules used in children have concluded that the validation studies for the PAS are of higher methodological quality, and that this score offers a superior diagnostic yield (sensitivity of 93% and negative predictive value of 10%), with level 2 evidence (rule broadly validated in multiple settings) according to the hierarchy of evidence for clinical prediction rules published by the Evidence-Based Medicine Working Group.5,32 Although at present it is recommended that the PAS be used with caution in clinical practice, since it does not achieve a diagnostic yield deemed sufficient,5,29,33 the literature also recognises its usefulness in the stratification into low- and high-risk groups of patients in who further diagnostic tests may then be rendered unnecessary,30,31,34 in guiding clinical decision making, and in improving the use of resources.5,35 The PAS is also useful as a tool for predicting appendicitis severity and the risk of complications, and can guide the decision to repeat a structured physical examination during the observation period.4,33
Variables in clinical prediction rules have rarely been replaced by other variables,36 and based on the reviewed literature, this has never been done in the PAS. Given that body temperature is of little use in differentiating between appendicitis and NSAP, we substituted the CRP level for it in the PAS. This modified PAS improved the diagnostic yield noticeably, as it distributed patients more appropriately in the different risk groups, although the difference was not statistically significant.
Some of the possible limitations of the study are: a) the impossibility of performing histopathological examination of the appendix, considered the diagnostic gold standard for appendicitis, in patients in the NSAP group (partial verification or workup bias).15,37 These unoperated patients are presumed to be NSAP cases, but epidemiology studies and observational studies that used imaging tests have demonstrated that cases of uncomplicated appendicitis may resolve spontaneously,38,39 so we cannot exclude that some cases categorised as NSAP were actually cases of resolved appendicitis. b) The heterogeneity of the NSAP group, with the potential inclusion of undetected cases of specific diseases (spectrum bias).37 We attempted to minimise this risk through the inclusion and exclusion criteria. c) The time elapsed between performance of laboratory tests and appendectomy (disease progression bias).37 To reduce this bias, we excluded cases where this interval exceeded 12h.
ConclusionsThe PAS may be helpful in the differential diagnosis of appendicitis and NSAP, correctly stratifying patients by risk of appendicitis. Tenderness in the RIF on coughing, hopping and/or percussion is the variable most strongly associated with appendicitis, which justifies its higher weight in the PAS score. Substituting the CPR level for the body temperature in this score would be advisable, as the latter lacks power to discriminate between NSAP and appendicitis. The CRP level, categorised based on the best cut-off point for the discrimination of appendicitis (25.5mg/L), could be used in its place, for while it exhibits only a moderate ability to differentiate between appendicitis and NSAP, when combined with the leukocyte and neutrophil counts, its value can be particularly useful in the diagnosis of appendicitis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Prada Arias M, Salgado Barreira A, Montero Sánchez M, Fernández Eire P, García Saavedra S, Gómez Veiras J, et al. Apendicitis versus dolor abdominal agudo inespecífico: evaluación del Pediatric Appendicitis Score. An Pediatr (Barc). 2018;88:32–38.
Previous presentation: This work will be submitted for consideration as an oral communication in the 65th Congress of the Asociación Española de Pediatría, Santiago, Spain, 2017.