The presence of apnoea in acute bronchiolitis (AB) varies between 1.2% and 28.8%, depending on the series, and is one of its most fearsome complications. The aim of this study is to determine the incidence of apnoea in hospitalised patients diagnosed with AB, and to define their associated risk factors in order to construct a prediction model.
Patients and methodA retrospective observational study of patients admitted to a tertiary hospital in the last 5 years with a diagnosis of AB, according to the classic criteria. Data was collected on the frequency of apnoea and related clinical variables to find risk factors in a binary logistic regression model for the prediction of apnoea. A ROC curve was developed with the model.
ResultsApnoea was recorded during the admission of 53 (4.4%) patients out of a total of 1.197 cases found. The risk factors included in the equation were: female (OR 0.6, 95% CI: 0.27–1.37), caesarean delivery (OR: 3.44, 95% CI: 1.5–7.7), postmenstrual age ≤43 weeks (OR: 6.62, 95% CI: 2.38–18.7), fever (OR: 0.33, 95% CI: 0.09–1.97), low birth weight (OR: 5.93, 95% CI: 2.23–7.67), apnoea observed by caregivers before admission (OR: 5.93, 95% CI: 2.64–13.3), and severe bacterial infection (OR: 3.98, 95% CI: 1.68–9.46). The optimal sensitivity and specificity of the model in the ROC curve was 0.842 and 0.846, respectively (P<.001).
ConclusionsThe incidence of apnoea during admission was 4.4 per 100 admissions of AB and year. The estimated prediction model equation may be of help to the clinician in order to classify patients with increased risk of apnoea during admission due to AB.
La presencia de apneas en la bronquiolitis aguda (BA) varía según las series entre el 1,2 y el 28,8%, y es una de sus complicaciones más temibles. Nuestro objetivo es conocer la incidencia de apneas en pacientes ingresados con diagnóstico de BA y definir sus factores de riesgo asociados para construir un modelo de predicción.
Pacientes y métodoEstudio observacional retrospectivo de los últimos 5 años de pacientes ingresados en un hospital terciario con diagnóstico de BA según los criterios clásicos. Se recogieron la frecuencia de apneas y las variables clínicas relacionadas, para encontrar factores de riesgo en un modelo de regresión logística binaria para la predicción de apneas. Para evaluar el modelo se elaboró una curva ROC.
ResultadosDe 1.197 casos, se registró apneas durante el ingreso en 53 (4,4%). Los factores de riesgo incluidos en la ecuación fueron: sexo femenino (OR 0,6; IC del 95%: 0,27-1,37), cesárea (OR: 3,44; IC del 95%: 1,5-7,7), edad posmenstrual ≤ 43 semanas (OR: 6,62; IC del 95%: 2,38-18,7), fiebre (OR: 0,33; IC del 95%: 0,09-1,97), bajo peso al ingreso (OR: 3,06; IC del 95%: 1,23-7,67), apneas antes del ingreso observada por los cuidadores (OR: 5,93; IC del 95%: 2,64-13,3) y sobreinfección bacteriana grave (OR: 3,98; IC del 95%: 1,68-9,46). La sensibilidad y la especificidad óptima del modelo en la curva ROC fueron de 0,842 y 0,846, respectivamente (p<0,001).
ConclusionesLa incidencia de apneas durante el ingreso fue de 4,4 por cada 100 ingresos de BA y año. La ecuación del modelo de predicción estimado puede ser de ayuda al clínico para clasificar a los pacientes con mayor riesgo de apnea durante el ingreso en la BA.
Acute bronchiolitis (AB) is the leading cause of hospitalisation for respiratory disease in children, and apnoea is one of its most worrisome complications in infants. It is a reason for hospital admission in itself, and often for admission to paediatric intensive care units (PICUs). In recent studies, the incidence of apnoea in these patients has ranged between 1.2% and 28.8%,1–4 although this broad variability is probably due to how apnoea is defined and the conflation of the apnoea subtypes investigated in different studies. Patients with AB may develop central, obstructive or mixed apnoeas. In young infants, obstructive apnoeas are clearly associated with the pathophysiology of bronchiolitis, where there is an increase in mucous secretions and inflammation of the airway. The mechanism by which AB produces apnoea is not well known, although it seems to be related to the facilitated release of GABA5 following infection and the stimulation of laryngeal chemoreceptors6,7 by the inflammatory response to the virus. The evidence also suggests that there is no difference between the different viruses that may cause AB in the incidence of apnoea,1 and the role in the development of apnoea of the frequent coinfections described in association with BA is still unclear.8
Several risk factors for the development of apnoea in the context of AB have been described, and the evidence is not uniform for all. The factors supported most strongly are preterm birth, younger age at diagnosis, absence of fever and low weight,1,3,9 while the results on prenatal or environmental exposure to tobacco, the duration of symptoms before admission, artificial formula feeding, decreased appetite or disease severity based on a variety of scales are not consistent across the published case series.1–3,10,11 Few studies have focused specifically on the development of apnoea in the context of AB,12 and we did not find a study in the literature that attempted to unify the various risk factors in a specific time-and-space frame with the aim of building a prediction model.
The potential availability of an instrument with an improved capacity to predict which infants are at higher risk of developing apnoea could help clinicians anticipate complications and optimise resources that may be limited in peak AB seasons, such as apnoea monitors, pulse oximeters, high-flow nasal cannulae or CPAP machines.
Our aim was to establish the incidence of apnoea in infants with AB during their stay in our hospital, and to define the risk factors associated with the development of apnoea for the purpose of building a prediction model and assessing its performance in a large series of patients.
Patients and methodWe conducted a retrospective observational analytical nested case-control study in a cohort of patients hospitalised with AB. We included patients admitted to a tertiary care referral hospital between January 2010 and March 2015 with a clinical diagnosis of AB based on the classical criteria proposed by McConnochie.13 We excluded patients with underlying chronic disease, such as congenital heart disease, neurologic disease with motor impairment, bronchopulmonary dysplasia, inborn errors of metabolism or congenital or acquired immunodeficiency. All our patients underwent antigen-detection testing for the presence of respiratory syncytial virus (RSV) on nasopharyngeal aspirate samples at admission following established procedure.14 We were able to conduct this study because AB is a frequent disease whose documentation has been standardised, so that the electronic health records of patients with AB include data on the variables under study recorded daily in a systematic and uniform way. Furthermore, all patients were monitored by pulse oximetry to detect abnormalities in their baseline oxygen saturation and/or heart rate until they were medically stable.
The researchers reviewed the electronic health records separately for the manual extraction of data on demographic and clinical characteristics, which was supplemented with an automated search for the response variable: apnoea during hospitalisation. In our protocol, apnoea is defined as any episode of cessation of breathing lasting 20s or longer, or a shorter respiratory pause associated with a decrease in oxygen saturation and/or heart rate. Patients with AB that had at least one episode of apnoea during their stay were considered cases, and the rest were considered controls. For each patient, we also collected data on sex, age, month of admission, birth weight, gestational age, postmenstrual age, singleton/multiple pregnancy, caesarean delivery, prenatal and postnatal exposure to tobacco, type of feeding, history of hypersensitivity reactions in a first-degree relative (defined as confirmed allergy, atopic dermatitis or asthma), duration of symptoms at admission (in days), fever, degree of decreased appetite, low weight below the 3rd percentile according to the WHO growth charts, severity according to a previously validated scale,15 and severe bacterial superinfection at admission, suspected on the basis of previously described clinical criteria16–18 (levels of C-reactive protein greater than 70mg/L or procalcitonin greater than 0.5ng/mL) or confirmed by blood or urine culture.
We used the licensed software SPSS 23.0 to perform the statistical analysis. We have expressed the results as proportions with 95% confidence intervals (CIs) for the primary outcome variable, and as median and interquartile range for quantitative explanatory variables. To evaluate potential risk factors for apnoea, we performed a bivariate analysis using the chi square test, comparing the primary outcome (apnoea during the stay) to each of the explanatory variables under study. We performed the multivariate analysis of the factors associated with the development of inpatient apnoea by means of binary logistic regression, manually adding variables corresponding to p-values of less than 0.25 in the bivariate analysis and variables shown to be clinically relevant in previous studies to the model one at a time. Variables with p-values of less than 0.25 that did not behave as confounders or interact with the other variables remained in the multivariate model, and the rest were excluded from it. We defined statistical significance as a p-value of less than 0.05. We plotted a theoretical sensitivity-specificity curve for the developed model: a receiver operating characteristic (ROC) curve. The probabilities calculated with the model for each patient based on the explanatory variables allowed us to predict the presence or absence of apnoea for different cut-off points of the estimated probability, which we compared with the actual presence of apnoea to calculate the area under the curve. We calculated the best cut-off point to maximise sensitivity and specificity, along with its positive and negative likelihood ratios.
We conducted the study after obtaining the approval of the ethics committee of our hospital.
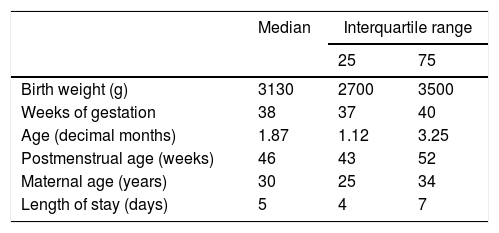
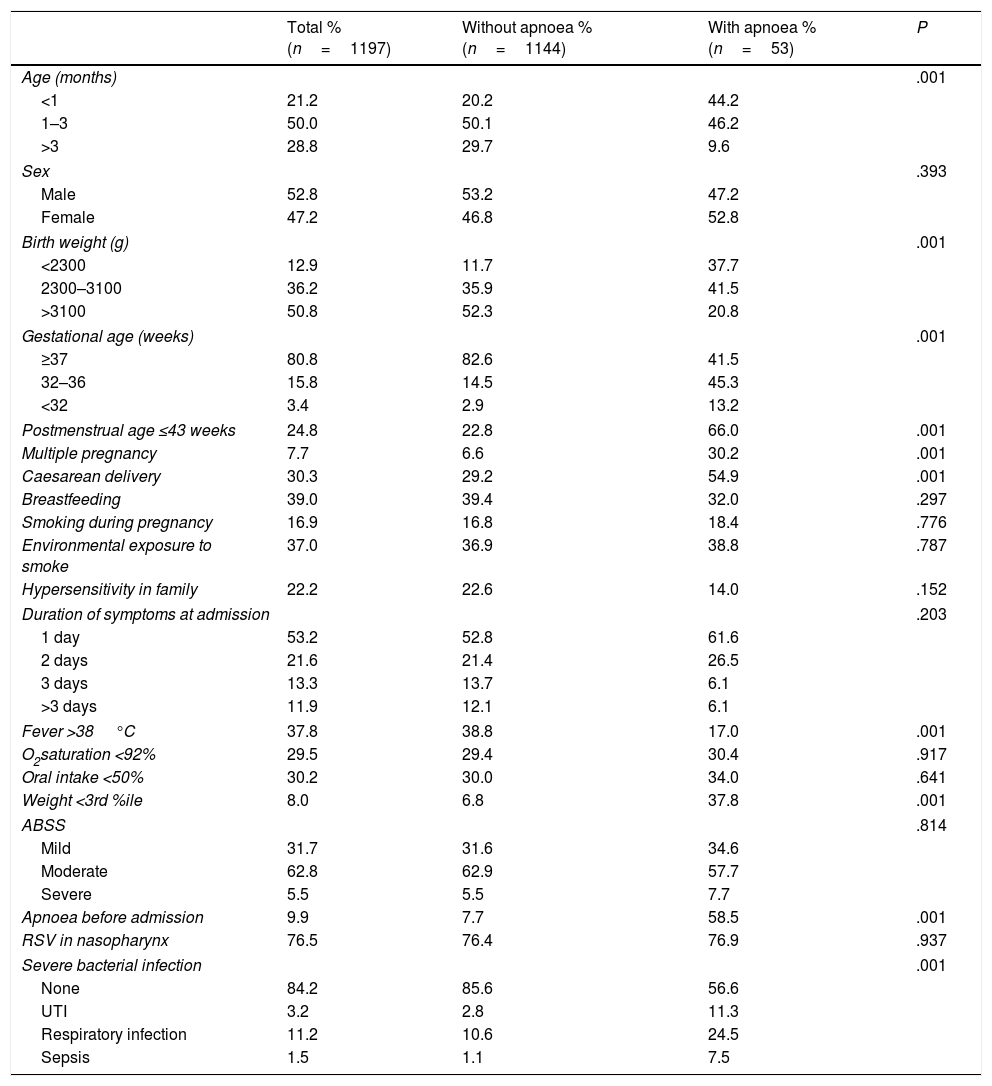
ResultsOf a total of 1.269 patients hospitalised with AB during the period under study, 1197 met the inclusion criteria. At least one episode of apnoea was detected during hospitalisation in 4.4% of cases of AB (53 patients; 95% CI, 3.3%–6.6%). Table 1 summarises the quantitative variables under study. Table 2 presents the distributions of the qualitative variables and the results of the unadjusted chi square test assessing the association of each of the clinical and demographic variables with the development of apnoea during hospitalisation.
Quantitative characteristics of the sample of hospitalised patients with acute bronchiolitis between 2010 and 2015 (n=1197).
| Median | Interquartile range | ||
|---|---|---|---|
| 25 | 75 | ||
| Birth weight (g) | 3130 | 2700 | 3500 |
| Weeks of gestation | 38 | 37 | 40 |
| Age (decimal months) | 1.87 | 1.12 | 3.25 |
| Postmenstrual age (weeks) | 46 | 43 | 52 |
| Maternal age (years) | 30 | 25 | 34 |
| Length of stay (days) | 5 | 4 | 7 |
Statistical significance of the presence of apnoea in patients with acute bronchiolitis in association with epidemiological and clinical variables (chi square).
| Total % (n=1197) | Without apnoea % (n=1144) | With apnoea % (n=53) | P | |
|---|---|---|---|---|
| Age (months) | .001 | |||
| <1 | 21.2 | 20.2 | 44.2 | |
| 1–3 | 50.0 | 50.1 | 46.2 | |
| >3 | 28.8 | 29.7 | 9.6 | |
| Sex | .393 | |||
| Male | 52.8 | 53.2 | 47.2 | |
| Female | 47.2 | 46.8 | 52.8 | |
| Birth weight (g) | .001 | |||
| <2300 | 12.9 | 11.7 | 37.7 | |
| 2300–3100 | 36.2 | 35.9 | 41.5 | |
| >3100 | 50.8 | 52.3 | 20.8 | |
| Gestational age (weeks) | .001 | |||
| ≥37 | 80.8 | 82.6 | 41.5 | |
| 32–36 | 15.8 | 14.5 | 45.3 | |
| <32 | 3.4 | 2.9 | 13.2 | |
| Postmenstrual age ≤43 weeks | 24.8 | 22.8 | 66.0 | .001 |
| Multiple pregnancy | 7.7 | 6.6 | 30.2 | .001 |
| Caesarean delivery | 30.3 | 29.2 | 54.9 | .001 |
| Breastfeeding | 39.0 | 39.4 | 32.0 | .297 |
| Smoking during pregnancy | 16.9 | 16.8 | 18.4 | .776 |
| Environmental exposure to smoke | 37.0 | 36.9 | 38.8 | .787 |
| Hypersensitivity in family | 22.2 | 22.6 | 14.0 | .152 |
| Duration of symptoms at admission | .203 | |||
| 1 day | 53.2 | 52.8 | 61.6 | |
| 2 days | 21.6 | 21.4 | 26.5 | |
| 3 days | 13.3 | 13.7 | 6.1 | |
| >3 days | 11.9 | 12.1 | 6.1 | |
| Fever >38°C | 37.8 | 38.8 | 17.0 | .001 |
| O2saturation <92% | 29.5 | 29.4 | 30.4 | .917 |
| Oral intake <50% | 30.2 | 30.0 | 34.0 | .641 |
| Weight <3rd %ile | 8.0 | 6.8 | 37.8 | .001 |
| ABSS | .814 | |||
| Mild | 31.7 | 31.6 | 34.6 | |
| Moderate | 62.8 | 62.9 | 57.7 | |
| Severe | 5.5 | 5.5 | 7.7 | |
| Apnoea before admission | 9.9 | 7.7 | 58.5 | .001 |
| RSV in nasopharynx | 76.5 | 76.4 | 76.9 | .937 |
| Severe bacterial infection | .001 | |||
| None | 84.2 | 85.6 | 56.6 | |
| UTI | 3.2 | 2.8 | 11.3 | |
| Respiratory infection | 11.2 | 10.6 | 24.5 | |
| Sepsis | 1.5 | 1.1 | 7.5 | |
ABSS, acute bronchiolitis severity scale; RSV, respiratory syncytial virus.
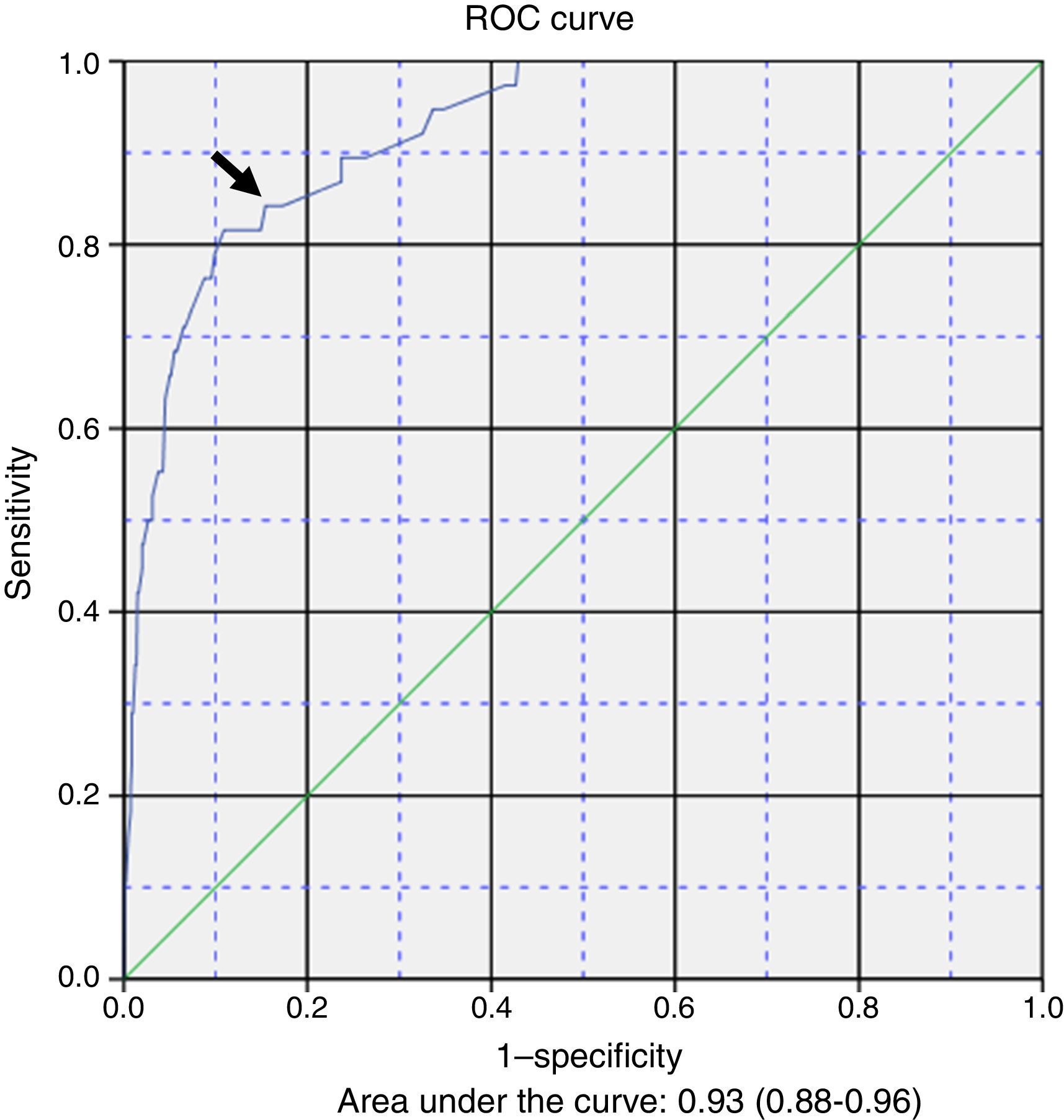
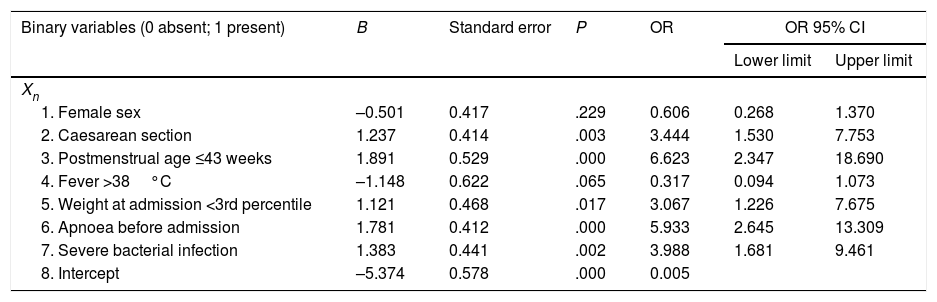
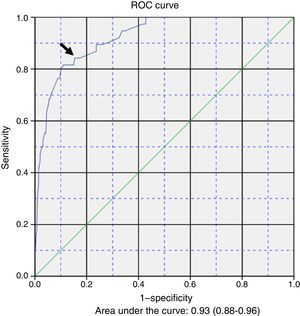
Table 3 presents the results of the multivariate logistic regression analysis used to develop a prediction model for the development of apnoea (Y) during hospitalisation. The risk factors (Xi) included in the equation were: 1) female sex; 2) caesarean delivery; 3) postmenstrual age ≤43 weeks; 4) fever; 5) low weight at admission; 6) caregiver-witnessed apnoea before admission, and 7) severe bacterial superinfection. The general equation for the logistic regression prediction model was: P(Y)=1/(1+exp(5.374+0.501X1−1.237X2−1.891X3+1.148X4−1.121X5−1.781X6−1.383X7). The p-value obtained in the Hosmer–Lemeshow test for goodness of fit was .907. Fig. 1 shows the ROC curve computed with the probabilities calculated with the model in relation to the actual cases of apnoea in our series. The area under the curve was 0.93 (CI, 0.88–0.96; P<.001). The optimal cut-off point estimated for the model corresponded to a p-value of .053356, a sensitivity of 0.842 and a specificity of 0.846. The positive likelihood ratio was 5.46 and the negative likelihood ratio was 0.18.
Binary logistic regression analysis to study the development of apnoea in infants with bronchiolitis during their hospital stay with the equation P(Y)=11+exp(5.374+0.501X1−1.237X2−1.891X3+1.148X4−1.121X5−1.781X6−1.383X7.
| Binary variables (0 absent; 1 present) | B | Standard error | P | OR | OR 95% CI | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Xn | ||||||
| 1. Female sex | –0.501 | 0.417 | .229 | 0.606 | 0.268 | 1.370 |
| 2. Caesarean section | 1.237 | 0.414 | .003 | 3.444 | 1.530 | 7.753 |
| 3. Postmenstrual age ≤43 weeks | 1.891 | 0.529 | .000 | 6.623 | 2.347 | 18.690 |
| 4. Fever >38°C | –1.148 | 0.622 | .065 | 0.317 | 0.094 | 1.073 |
| 5. Weight at admission <3rd percentile | 1.121 | 0.468 | .017 | 3.067 | 1.226 | 7.675 |
| 6. Apnoea before admission | 1.781 | 0.412 | .000 | 5.933 | 2.645 | 13.309 |
| 7. Severe bacterial infection | 1.383 | 0.441 | .002 | 3.988 | 1.681 | 9.461 |
| 8. Intercept | –5.374 | 0.578 | .000 | 0.005 | ||
Y: apnoea; Xn: risk factor.
In our study, the incidence of inpatient apnoea in patients hospitalised with bronchiolitis during the season was 4.4%, consistent with the figures reported in one of the largest published studies focused on the development of apnoea in the context of AB.1 Recent studies in the Mediterranean region have found similar results.12 Several studies have reported that the incidence of recurrent inpatient apnoea in patients hospitalised with AB is distinctly lower than the incidence of apnoea reported by caregivers seeking care in emergency departments.1,3,11 The proportion of cases in our series where possible episodes of apnoea before admission were reported by caregivers was 9.9% (119 patients). In the study mentioned above,1 the number of patients that may have had episodes of apnoea prior to admission reached 15.6%, somewhat higher than the proportion found in our study. This risk factor has been described previously in the literature, with differing OR values.1,3,11 Although the issue is broached with caution, most studies on apnoea recommend taking into account the descriptions made by parents and consider reports of potential episodes witnessed prior to admission as actual episodes of apnoea, with a careful interpretation on the part of health care providers.1,3 Since episodes of apnoea are less frequent during hospitalisation, it is possible that patients managed in outpatient settings that do not receive the respiratory care offered at hospital (frequent suctioning of secretions, nebuliser treatment, postural drainage, oxygen therapy, etc.) develop predominantly obstructive apnoeas that do not recur once the patient is hospitalised. It is likely that episodes in hospitalised patients are mostly of central apnoea, as these patients are monitored continuously and undergo frequent suctioning of secretions. This subtype of apnoea has the highest associated morbidity and is more likely to require intensive care.9 As was the case in our series, previously published prospective studies have concluded that the risk of apnoea does not seem to be associated with the specific virus that causes the disease or even by the presence of viral coinfection.1
As for the risk factors identified by logistic regression, we ought to highlight, first of all, postmenstrual age, which we analysed with a threshold of 43 weeks, the point traditionally set as the limit for the development of apnoea in newborns, finding an OR of 6.6 for postmenstrual age of 43 weeks or greater. The protection conferred by increasing age is associated with the level of maturity of the infant and is the factor described most frequently in the literature.1,10,11 Another risk factor that has been widely reported is low weight below the 3rd percentile. It would be logical to assume that the nutritional status of the infant would influence his or her overall maturity. Therefore, it would theoretically make sense to consider adequate nutrition a means to protect against the development of apnoeas in infants.
Fever in the context of AB has been previously described as a protective factor against the development of apnoea.9,10 In our series, the p-value for fever as a protective factor was near the threshold for statistical significance (Table 3). A larger sample may have found evidence of the protective effect of fever on the development of apnoea as described by other authors. Fever is a defensive response to infection, and it is known that hypothermia is associated with a higher incidence of apnoea.11
We have not found published data on the association between the development of apnoea and a history of caesarean delivery, which was identified as a risk factor in our series (Table 3). In published case series of hospitalised patients with AB with a size similar to ours,1,2,12 this variable was not included in the analyses. This is therefore a novel finding that may be related to differences in the maturation process in infants delivered vaginally, as in this type of delivery these is a more efficient clearance of amniotic fluid from the airways as well as differences in the initial colonisation. There is growing evidence on the significant role of the microbiota in various respiratory illnesses. The microbiota present in the respiratory tract may influence the outcomes of RSV infection by modulating the immune response, as has been observed in other respiratory diseases.19,20 In term or late preterm infants, born between 37 and 39 weeks’ gestation, caesarean delivery increases the risk of respiratory distress syndrome.21 Furthermore, in many instances, preterm newborns delivered by elective caesarean section have been exposed to antenatal steroids with the purpose of inducing lung maturation, which may have an impact on future respiratory outcomes, although we were unable to collect data on this factor in our study.
Lastly, severe bacterial superinfection, which we restricted to 3 categories in our series (urinary tract infection, respiratory superinfection with findings indicative of occult bacteraemia and sepsis), was significantly associated as an independent risk factor with the presence of apnoea. At the mean age of the patients included in the study (2.4 months), these infections can in fact be the cause of apnoea of infancy. Therefore, clinicians should consider the possibility of bacterial superinfection in any infant with AB that develops apnoea.
The probabilities of having episodes of apnoea during hospitalisation, calculated based on our logistic regression model and compared to the actual presence or absence of apnoea by means of a ROC curve, allowed us to obtain an acceptable cut-off point in the top left corner. While this prediction model was constructed with clinical variables, it seems that it could be an acceptable tool for clinicians, with useful positive and negative likelihood ratios.
The main strength of the study is the mining of the standardised electronic health records of a large series of patients managed under a systematic treatment and monitoring protocol. On the other hand, one of its limitations is that it was restricted to a single tertiary care referral hospital. The study also did not take into account the aetiology of AB or the presence of coinfection. The ROC curve and its sensitivity and specificity values are theoretical calculations based on sample data and performance of further research for confirmation, preferably prospective multicentre studies, would be desirable in order to improve the performance of the model.
ConclusionsApnoea was detected during the hospital stay in 4.4% of cases of AB per year. The independent factors included in our prediction model were postmenstrual age ≤43 weeks, caesarean delivery, fever, low weight at admission, caregiver-witnessed apnoea previous to admission and severe bacterial infection. A prediction model equation that includes these variables may help clinicians identify patients at higher risk of apnoea in order to remain alert and anticipate the need for resources.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ramos-Fernández JM, Moreno-Pérez D, Gutiérrez-Bedmar M, Ramírez-Álvarez M, Martínez García Y, Artacho-González L, et al. Apneas en lactantes con bronquiolitis: incidencia y factores de riesgo para un modelo de predicción. An Pediatr (Barc). 2018;88:160–166.