The factors and patterns associated with antibiotic consumption in infants are unclear. Our aim was to assess the cumulative incidence of antibiotic consumption from birth to 16 months and identify factors associated with antibiotic consumption among infants aged 4–16 months.
Material and methodsWe conducted a cross-sectional study in 2016 in a sample of 18 882 women from Galicia, Spain, who had given birth to a live child between September 1, 2015 and August 31, 2016. We calculated the cumulative incidence of antibiotic consumption based on maternal reports regarding the infant’s consumption from birth to 14 months obtained through interviews; we did not estimate consumption at ages 15 and 16 months due to the small sample size. To assess which factors were associated with antibiotic consumption, we carried out a nested case-control study matching cases and controls for birth month on a 1:1 ratio.
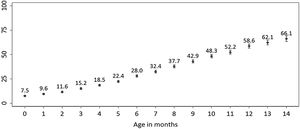
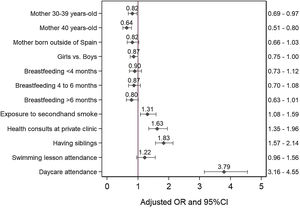
ResultsThe cumulative incidence of antibiotic consumption among infants aged 0–14 months increased from 7.5% to 66.0%. The case-control study included data for 1852 cases and 1852 controls. Daycare attendance (OR: 3.8 [95% CI: 3.2–4.6]), having older siblings (OR: 1.8 [95% CI: 1.6–2.1]), health care visits to private clinics (OR: 1.6 [95% CI: 1.4–2.0]), and passive smoking (OR: 1.3 [95% CI: 1.1–1.6]) were associated with an increased probability of antibiotic consumption. Maternal age between 30–39 years or 40 years and over at the time of birth was associated with a decreased probability of antibiotic consumption (OR: 0.8 [95% CI, 0.7–1.0] and OR: 0.6 [95% CI: 0.5–0.8], respectively).
ConclusionsSome of the factors associated with antibiotic consumption in infants are modifiable and should be considered in the development of public health measures aimed at reducing antibiotic consumption.
Los factores y patrones asociados al consumo de antibióticos en los lactantes no están claros. Nuestro objetivo fue evaluar la incidencia acumulada de consumo de antibióticos desde el nacimiento hasta los 16 meses e identificar los factores asociados al consumo de antibióticos entre lactantes de 4 a 16 meses.
Material y métodosSe realizó un estudio transversal en 2016 que incluyó una muestra de la población de 18.882 mujeres españolas de Galicia que habían dado a luz a un niño vivo entre el 1 de septiembre de 2015 y el 31 de agosto de 2016. Se calculó la incidencia acumulada de consumo de antibióticos a partir de los resultados de la entrevista a la madre sobre el consumo del lactante desde el nacimiento hasta los 14 meses; no se estimó a los 15 y 16 meses debido al reducido tamaño muestral. Para valorar las características asociadas al consumo de antibióticos se anidó en el estudio transversal un estudio de casos y controles emparejando por mes de nacimiento, un control por caso.
ResultadosLa incidencia acumulada de consumo de antibióticos entre los lactantes de 0 a 14 meses de edad aumentó del 7,5% al 66,0%. Para el estudio de casos y controles, se obtuvo información de 1.852 casos y de 1.852 controles. La asistencia a la guardería (OR: 3,8 [IC 95%: 3,2–4,6]), tener hermanos/as mayores (OR: 1,8 [IC 95%: 1,6–2,1]), las consultas sanitarias en la clínica privada (OR: 1,6 [IC 95%:1,4–2,0]) o haber estado expuesto al humo ambiental de tabaco (OR: 1,3 [IC 95%: 1,1–1,6]) se asociaron con un mayor riesgo de consumo de antibióticos. Tener madres de entre 30 y 39 años o de 40 años y más en el momento del parto se asoció con un menor riesgo de consumo de antibióticos (OR: 0,8 [IC 95%: 0,7–1,0] y OR: 0,6 [IC 95%: 0,5–0,8], respectivamente).
ConclusionesAlgunos de los factores asociados al consumo de antibióticos en los lactantes pueden ser modificables y deben ser considerados en el desarrollo de medidas de salud pública dirigidas a reducir el consumo de antibióticos.
Antimicrobial resistance, associated with the indiscriminate and sometimes inappropriate use of antibiotics, constitutes an important public health problem due to its role in decreasing the therapeutic effectiveness of antibiotics.1 The development of antibiotic resistance is outpacing the development of new antibiotic agents by the pharmaceutical industry.2
Antimicrobial consumption data from the 2021 European Centre for Disease Prevention and Control survey shows that 20 out of 1000 inhabitants (2%) in Spain were receiving antibiotics each day in 2021, placing Spain among the 7 European countries with the highest consumption.3 A previous study found striking differences in the use of these drugs between Nordic and Mediterranean countries and even substantial variation within different geographical areas.4 Overall, the community consumption of antibiotics for systemic use in Europe decreased between 2019 and 2020, concomitant with the COVID-19 pandemic, particularly in Greece, Poland, Spain, Luxembourg, the Slovak Republic and France.5
In 2017, the second line of strategy established in the Plan Nacional frente a la Resistencia a los Antibióticos (PRAN, National Plan against Antibiotic Resistance) prioritized the need to reduce the overall prescription of antibiotics in Spain, particularly in the paediatric population and for diseases without a bacterial aetiology.6 Antibiotics are among the drugs most commonly prescribed to children, but approximately 20%–50% of these prescriptions are for management of nonbacterial upper respiratory tract infections, for which these drugs are not effective.7–9 Moreover, a large number of prescriptions are for broad-spectrum agents, with no consideration of the substantial seasonal variation, clinical guidelines or scientific evidence, which reflects inappropriate antibiotic prescribing practices.4,10,11
Since the introduction of high-valency pneumococcal conjugate vaccines (PCV10 and PCV13) in childhood vaccination programs and the implementation of measures focused on limiting antibiotic prescribing to medically-appropriate conditions, the use of antibiotics among children has declined worldwide.12–14 Previous studies conducted in the paediatric population in Spain showed a decrease in antibiotic consumption since 2016.15–17 A study conducted in Albacete showed that the prevalence of antibiotic use decreased between 2017 and 2019 among children aged 0–14 years (38.9% vs 35.2%) and was higher in the first 4 years of life.17 Another study found a consumption of 11.9 defined daily doses of antibiotics per 1000 inhabitants per day (1.2) in the paediatric population of Asturias in 2018 compared to 14.7/1000/day (1.5) in 2016.16
Antibiotic consumption among infants may be affected by physicians’ and parents’ attitudes, social and cultural factors, health disparities, parental sociodemographic characteristics or certain traits and behaviours in the child.18,19 However, the current evidence on the factors that may lead to antibiotic consumption in early life is still scarce for many geographical areas.
The aim of the study was to estimate the cumulative incidence of antibiotic consumption among infants from birth to age 16 months in Galicia, Spain, and to identify the factors associated with antibiotic consumption in this population.
Material and methodsStudy setting and sampleWe conducted a cross-sectional survey of women residing in the autonomous community of Galicia (in north-western Spain) who were at least 18 years of age and had given birth to a live child between September 1, 2015 and August 31, 2016 (N = 18 882) in 2016. The study was carried out in the framework of the Risk Behaviours Surveillance System (SICRI, Sistema de Información sobre Conductas de Riesgo). We recruited participants through the register of the Programme for the Early Detection of Endocrine and Metabolic Diseases in the Neonatal Period. We used stratified random sampling to select a representative sample of mothers by age group (18–24; 25–29; 30–34; 35–39; 40 and older) (Supplementary Table 1). The sample size was calculated independently for each age stratum, with an expected prevalence of 50%, and an error of less than or equal to 2% with a 95% confidence interval (95% CI).
The study included all the children born to the selected mothers within the established timeframe, with the exception of those product of multiple gestations, in which case only one child was selected at random. At the time of the survey, children were aged 4–16 months.
Sources of informationWe determined the consumption of antibiotics based on the maternal responses to the survey. Mothers were asked whether their children had taken antibiotics at least once and, in the case of an affirmative answer, the age of first use. We collected the information through a computer-assisted telephone interviewing (CATI) system. This software system provides the user (interviewer) with scripted instructions for collecting specific information and entering it into the computer. The computer then automatically displays the next question in a structured protocol based on the participant’s response. In addition, all the collected data collected is dumped into a central system so that it can be extracted for further analysis.20 The questionnaire included several questions regarding maternal behaviours, attitudes and practices associated with antibiotic consumption in the previous literature. We collected the following information on the mother in reference to the 6 months preceding the detection of the pregnancy, the pregnancy period, the time of delivery and the time of the survey: age group (age at delivery: 18–29, 30–39, ≥40 years and over), educational attainment (basic: mother who could not read or write, had no schooling, or did not complete primary education; medium: completed secondary/high school education; high: university degree), employment status (employed: actively employed or on leave; unemployed/not working; inactive: student, homemaker not seeking employment, pensioner/retired/pre-retired or another reason), country of birth, and tobacco use during pregnancy.
We also collected data regarding the personal and sociodemographic characteristics of the child from birth till age 16 months: sex assigned at birth, type of delivery (vaginal, caesarean), preterm birth (gestational age <37 weeks), small for gestational age (birth weight <10th percentile), post-birth hospitalization beyond the routine postnatal stay, assessment of health status at birth as reported by the mother (very good/good, regular, poor/very poor), breastfeeding duration (never, <4 months, 4−6 months, >6 months), pacifier/dummy use, passive smoking (PS) on account of maternal smoking or exposure through other smokers, as reported by the mother, having older siblings, childcare centre/nursery attendance, participation in swimming lessons, visits to private clinics compared to exclusive use of public health care system for check-ups.
To assess the factors associated with antibiotic consumption, we carried out a nested case-control study including 1 control per case. The definition of case was a child aged between 4 and 16 months whose mother reported, at the time of the survey, that they had taken antibiotics between these ages. We defined control as a child who had never taken antibiotics. We ought to highlight that we excluded infants aged less than 4 months from this analysis because the latter included variables like childcare centre attendance or swimming lessons among the factors that could affect antibiotic consumption.
In the case group, we assessed breastfeeding, pacifier use, childcare centre attendance and swimming at the time of taking antibiotics for the first time. In the control group, we assessed the same information for the time the child was the same age as the paired case at the first exposure to antibiotics.
Data analysisWe calculated the cumulative incidence of antibiotic consumption for each month since birth, based on the age of the first use of antibiotics. The percentage represented children aged at least X months who had already consumed antibiotics by month X of life. We also calculated 95% confidence intervals (CIs) for these percentages. We did not estimate the cumulative incidence of antibiotic consumption for the 15- and 16-months age groups due to their small size (487 and 179 children, respectively) (Supplementary Table 2).
In the nested case-control study, we selected one control for each case matched for the date of birth (or the closest date of birth) to ensure the control was as close as possible in age to the the case at the time the latter received antibiotics. This analysis excluded infants younger than 4 months (n = 79) or children who had consumed antibiotics before age 4 months (n = 951), children who had attended a childcare centre and later withdrawn from it (n = 64), and children for whom we were unable to obtain information regarding antibiotic consumption (n = 2). Lastly, another 255 children were excluded from the analysis because of a lack of controls that could be matched by month of birth, so the final sample included a total of 1852 cases and 1852 controls.
We fitted a conditional logistic regression model to establish the association between the various mother and child characteristics and antibiotic consumption. A bivariate analysis was performed to estimate the unadjusted odds ratio (ORs) of taking antibiotics for each categorical independent variable (maternal age group, maternal educational attainment, mother born outside Spain, maternal smoking during pregnancy, child sex, type of delivery, prematurity, small for gestational age, post-birth hospitalization, health status at birth, breastfeeding, pacifier use, passive smoking in child, child care centre attendance, swimming lesson attendance, visits to private clinics and having siblings). Subsequently, we fitted a multivariable model to assess the impact of all the selected variables on antibiotic consumption. The variables included in the final model (maternal age group, mother born outside of Spain, child sex, breastfeeding, PS in child, childcare centre attendance, swimming, visits to private clinics and having siblings) were selected taking into account their relevance and the statistical significance of the association (P value less than or near 0.05). Finally, all selected variables had P values of less than 0.1. We calculated 95% CIs for the ORs.
The statistical analysis was performed in the weighted sample using the Stata software package, version 14.2.
Ethical considerations and informed consentThe study was conducted in adherence to the Declaration of Helsinki and national and institutional legislation and regulations regarding clinical research and personal data protection in Spain. Participation in the study was voluntary and anonymous, and full confidentiality was guaranteed. The study was conducted by telephone, so that agreement to participate implied consent.
ResultsA total of 6436 women aged 18–49 years agreed to participate in the study, of who 71.3% were aged 30 years or older. A total of 10 771 telephone calls were made for the study, of which 6436 yielded interviews with participants, 1998 refusals to participate in the study and the rest were failed contacts (non-existent numbers). Therefore, the response rate, calculated as the percentage of women interviewed over those contacted, was 76%.
We obtained information regarding 6436 children aged 0–16 months at the time of the interview with the mother. Of this total, 48.8% (95% CI, 47.5%–50.1%) had received antibiotics at least once (Supplementary Table 3). The cumulative incidence of antibiotic consumption over the first 14 months of life increased with each month of age, from 7.5% at 0 months to 66.1% at age 14 months (Fig. 1). Independently of age, the cumulative incidence of antibiotic consumption was greater in infants whose mothers were less than 40 years old compared to those with mothers aged 40 or more years.
The nested case-control study included 1852 cases and 1852 controls. Overall, 22% of the children were aged less than 6 months old, 39% were 6–8 months old, 26% were 9–11 months old and 13% were 1 year of age or older. Table 1 summarises the characteristics of mothers and children in the case and control groups, along with the unadjusted OR for each of the studied variables.
Odds ratio (OR) of taking antibiotics according to mother and child characteristics. Bivariate analysis.
| Cases (n = 1852) | Controls (n = 1852) | Antibiotic use | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | OR | 95% CI | P | |
| Maternal age (years) | |||||||||
| 18–29 | 535 | 21.1% | 19.9%–22.3% | 511 | 20.6% | 19.3%–21.8% | 1 | ||
| 30–39 | 1036 | 70.0% | 68.6%–71.4% | 998 | 68.1% | 66.6%–69.6% | 0.99 | 0.86–1.15 | 0.938 |
| ≥40 | 281 | 8.9% | 8.1%–9.7% | 343 | 11.3% | 10.5%–12.2% | 0.79 | 0.65–0.96 | 0.018 |
| Maternal educational attainment | |||||||||
| Basic | 415 | 20.1% | 18.2%–21.9% | 387 | 18.7% | 16.9%–20.5% | 1 | ||
| Medium | 678 | 37.1% | 34.7%–39.4% | 669 | 35.8% | 33.4%–38.1% | 0.95 | 0.80–1.12 | 0.524 |
| High | 759 | 42.9% | 40.5%–45.2% | 796 | 45.6% | 43.2%–48.0% | 0.89 | 0.75–1.05 | 0.180 |
| Mother born outside of Spain | 200 | 10.3% | 8.9%–11.8% | 236 | 12.4% | 10.8%–14.0% | 0.83 | 0.67–1.01 | 0.064 |
| Mother smoked during pregnancy | 251 | 13.2% | 11.6%–14.8% | 214 | 10.7% | 9.3%–12.2% | 1.21 | 0.99–1.48 | 0.061 |
| Sex of child | |||||||||
| Male | 983 | 53.3% | 50.9%–55.7% | 894 | 47.7% | 45.3%–50.1% | 1 | ||
| Female | 869 | 46.7% | 44.3%–49.1% | 958 | 52.3% | 49.9%–54.8% | 0.83 | 0.73–0.94 | 0.004 |
| Type of delivery | |||||||||
| Vaginal | 1394 | 75.1% | 72.9%–77.2% | 1394 | 74.9% | 72.8%–77.0% | 1 | ||
| Caesarean | 458 | 25.0% | 22.8%–27.1% | 458 | 25.1% | 23.0%–27.2% | 1.00 | 0.86–1.16 | 1.000 |
| Preterm birth | 118 | 7.3% | 5.9%–8.6% | 128 | 7.1% | 5.8%–8.4% | 0.92 | 0.71–1.19 | 0.517 |
| Small for gestational age | 157 | 8.8% | 7.4%–10.3% | 164 | 8.2% | 6.9%–9.5% | 0.95 | 0.76–1.20 | 0.681 |
| Post-birth hospitalization | 223 | 12.7% | 11.0%–14.4% | 228 | 12.8% | 11.1%–14.4% | 0.98 | 0.80–1.18 | 0.805 |
| Assessment of health status at birth | |||||||||
| Very good | 943 | 50.3% | 47.8–52.7% | 954 | 52.0% | 49.6%–54.5% | 1 | ||
| Good | 693 | 37.8% | 35.5%–40.2% | 691 | 36.8% | 34.5%–39.1% | 1.01 | 0.88–1.17 | 0.845 |
| Normal | 168 | 9.2% | 7.8%–10.7% | 164 | 8.7% | 7.3%–10.0% | 1.04 | 0.82–1.30 | 0.767 |
| Poor–very poor | 48 | 2.7% | 1.9%–3.5% | 43 | 2.5% | 1.7%–3.3% | 1.14 | 0.74–1.76 | 0.562 |
| Breastfeeding | |||||||||
| Never | 374 | 19.0% | 17.1%–20.9% | 323 | 17.2% | 15.4%–19.0% | 1 | ||
| <4 months | 469 | 25.2% | 23.1%–27.3% | 438 | 22.5% | 20.5%–24.5% | 0.93 | 0.76–1.13 | 0.455 |
| 4–6 months | 585 | 32.1% | 29.9%–34.4% | 611 | 33.4% | 31.1%–35.7% | 0.84 | 0.69–1.02 | 0.077 |
| >6 months | 424 | 23.7% | 21.6%–25.8% | 480 | 26.9% | 24.7%–29.1% | 0.75 | 0.61–0.92 | 0.006 |
| Pacifier/dummy use | 1521 | 81.9% | 80.0%–83.8% | 1472 | 79.3% | 77.3%–81.2% | 1.18 | 1.00–1.39 | 0.047 |
| Current child exposure to second-hand smoke | 396 | 20.7% | 18.7%–22.6% | 322 | 16.8% | 15.0%–18.6% | 1.30 | 1.10–1.54 | 0.002 |
| Childcare centre attendance | 726 | 40.7% | 38.3%–43.1% | 315 | 18.3% | 16.3%–20.2% | 3.57 | 2.99–4.25 | <0.001 |
| Swimming lessons | 191 | 11.6% | 10.0%–13.2% | 172 | 10.2% | 8.7%–11.8% | 1.12 | 0.90–1.40 | 0.294 |
| Visits to private clinic | 449 | 24.4% | 22.3%–26.5% | 307 | 16.8% | 15.0%–18.6% | 1.64 | 1.39–1.94 | <0.001 |
| Having siblings | 930 | 52.0% | 49.6%–54.4% | 729 | 40.2% | 37.9%–42.6% | 1.57 | 1.38–1.80 | <0.001 |
Fig. 2 presents the adjusted OR for each of the independent variables. The risk of antibiotic use was lower in children whose mothers were aged more than 30 years. Having older siblings, passive smoking in the child, childcare centre attendance and visiting private clinics for check-ups were associated with an increased probability of antibiotic consumption.
DiscussionThe results of our study show that more than half of children aged up to 16 months in Galicia had taken antibiotics within 12 months of birth. Antibiotic consumption in the first year of life was associated with maternal age less than 30 years, having older siblings, passive smoking, childcare centre attendance and visiting private clinics for routine child checkups. Although these risk factors have been identified in previous studies, they have never been assessed together in order to quantify the contribution of individual variables contribution to the overall risk. This characterization can allow the identification of modifiable factors on which to focus efforts toward decreasing the rate of antibiotic use.
Inappropriate antibiotic use in early life may not only lead to personal harm but may also contribute to bacterial resistance, which has emerged as one of the public health threats of the 21st century. Although a statistically significant decrease was observed in most Western countries over the 2011–2020 period due to different initiatives promoting the rational use of antibiotics, especially in infants,5,21–23 substantial differences exist between countries and even within regions, and differences of up to 7.5-fold has been observed in paediatric antimicrobial use across several industrialized countries.24,25 One major challenge in tackling this problem is identifying the areas at high risk and potential factors that may contribute to this risk. Our study shows that antibiotic consumption in the autonomous community of Galicia (Spain) is much higher than the estimated consumption in other European countries.18,26 Our study corroborated the findings of previous studies that had already identified Spain one of the countries in the European Union with the greatest antibiotic consumption.3
An interesting finding from this study is that maternal age may affect antibiotic prescription. This is consistent with previous research that has also found a decrease in antibiotic use with increasing maternal age.19 While the exact reason for this finding is unknown, it could be related to the fact that greater maternal age is commonly associated with increased psychosocial well-being and higher socioeconomic status. The results of a cross-sectional survey carried out in Queensland showed that older mothers were more likely to report being optimally informed and rapport with care providers.27
Our results also seem to suggest that breastfeeding may have a protective effect against antibiotic consumption, but this association is uncertain because it did not remain significant in the final model. Breastfeeding has been associated with benefits for both mother and child (increased resistance to infection, lower risk of obesity or decreased risk of allergy) in other studies.28 However, while the prophylactic effect of breastfeeding against gastrointestinal infections has been clearly demonstrated in developed countries, its benefits against respiratory tract infections are under debate.29–31 Some authors argue that the fact that it is not always possible to differentiate between mixed and exclusive breastfeeding may have affected the results.32
On the other hand, we found that PS constitutes a modifiable risk factor for antibiotic consumption during the first year of life. In agreement with other studies performed in infants aged less than 1 year, there was an increased probability of antibiotic consumption both among infants exposed to second-hand smoke and among those whose parents were smokers.18 Passive smoking is a known risk factor for lower respiratory tract and middle-ear infections.33 The infant population is especially vulnerable to the adverse effects of PS due to anatomical and physiological characteristics, such as smaller bronchial tubes, a faster respiratory rate and undeveloped immune systems.34 A systematic review and meta-analysis of 60 studies that assessed the risk factors for lower respiratory tract infections in the first 2 years of life concluded that passive smoking is a major risk factor for lower respiratory tract infections, with risk increasing with exposure, especially in the case of postnatal maternal smoking.35 In our study, 2% of mothers reported that their infants, aged less than 1 year, spent time in places where they were exposed to tobacco smoke.
Childcare centre attendance and having older siblings were also found to be risk factors for antibiotic use in our study. It has been previously suggested that children with older siblings might suffer from earlier respiratory infections compared to first-born children; however, their immune system might also mature earlier, providing them with improved resistance against infections later in childhood.36 When it comes to biological sex, it has been proposed that boys are at higher risk of respiratory infections and, therefore, antibiotic consumption, since their airways are narrower.37 Our results suggest that the risk of antibiotic use could be lower among female infants; however, this association is uncertain because it did not remain significant in the final model.
Furthermore, we also observed that the choice of private over public health care facilities for infant checkups was associated with an increased consumption of antibiotics in the first year of life. In our study, 24.4% of antibiotic users had their checkups at a private clinic. Some mothers may prefer to take their children to private health clinics when they are sick, compared to public clinics, due to the faster and easier access (no waiting list), the possibility of choosing the provider and the desire to get a diagnosis and treatment in a shorter time frame.38 A previous systematic review conducted in Africa also found an increased prescription of antibiotics in private facilities compared to public ones (51.3% vs 45.0%, respectively).39 Medical practitioners working in the private sector may be more likely to prescribe antibiotics due to potential financial conflicts of interest in relation to pharmaceutical industries, a fear of losing customers who seek antibiotics or excessive caseloads and time constraints which that could affect appropriate decision-making.39–41
Antibiotic consumption in the paediatric population of Galicia has probably decreased in recent years, especially after the COVID-19 pandemic, based on a recent study conducted in Spain.42 This trend could be due to the reduction in infectious respiratory diseases after the implementation of strict measures by the Spanish government during the SARS-CoV2 pandemic (closure of educational centres, businesses, and non-essential activities, home confinement, and mandatory use of masks); another possible reason could be an increased public awareness of the rational use of antibiotics.6,42 Unfortunately, this could not be verified in our study due to the lack of recent data.
This study has several strengths and limitations. The main strength is the sample size, as it accounts for more than 1 in 3 of the 19 000 mothers who gave birth in Galicia during the studied period. Additional strengths were that the sample was representative of the population and the year-round data on births. Moreover, we collected data on multiple variables potentially related to antibiotic consumption that had been previously identified in different studies, but not assessed together. In the case-control study, to avoid the confounding effect of the season of birth on the risk of antibiotic prescription, cases and controls were matched by birth month.19
The main limitation involves the cross-sectional design of the study and the presence of biases intrinsic to maternal self-reporting (information bias) and questions referring to past exposure (recall bias). We are aware of the potential issues that may arise in calculating a cumulative incidence using a cross-sectional study design. Yet, it should be taken into account that this is the first study that has embedded a nested matched case-control study to examine factors associated with antibiotic consumption in the Spanish paediatric population. Moreover, this study captures data on antibiotic consumption practices from the moment of birth to the age of the child in months (4–16 months) over an uninterrupted 1-year period. While recall bias is a concern, the interval of recall was relatively short (4–16 months). Moreover, it is unlikely that temporal trends could have influenced the incidence estimated in this sample, given the short study period (12 months). Another limitation of the study is that it relied on self-reported information. This becomes important in relation to certain variables, such as tobacco consumption or the exposure of the child to second-hand smoke, as social bias would encourage mothers to under-report. Information was self-reported by mothers and misclassification could be present, but we have no reason to suspect either systematic or differential measurement error associated with antibiotic consumption, as mothers were not aware of the hypothesis being tested.
Although Streptococcus pneumoniae is considered a frequent cause of infection in children under 3 years, we did not include vaccination status in the analysis because previous studies estimated a very high vaccination coverage among Spanish children after the inclusion of the pneumococcal conjugate vaccine in the official paediatric vaccination schedule.7 Moreover, 98.8% of the Galician mothers who participated in the study reported that their children had received all the vaccines recommended by the paediatrician.
We are aware that from the point of view of epidemiological surveillance, it would have been interesting to obtain information on the drug prescribed, the healthcare setting where it was prescribed (hospital or community/day clinic) or the disease of the child. However, this was not the aim of this particular study.
In summary, the results of the study show that antibiotic consumption during the first year of life is substantially high in the infant population of Galicia. The study provides useful information regarding the factors associated with an increased antibiotic consumption in infants. This information could be useful toward designing tailored interventions and campaigns to decrease antibiotic use in many countries that, like Spain, have a substantial consumption.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflicts of interest to disclose.