In 2009, two cases of seizures in adolescents following quadrivalent human papillomavirus vaccine (qHPV) administration generated important media attention, and adversely affected public trust in this vaccine. Our objectives were to describe suspected adverse reactions (SARs) reported to the Pharmacovigilance Centre in the Valencian Community (PCVC) after administration of HPV vaccine, and to compare reporting rates of syncope and seizures following this vaccine with those of other vaccines administered to girls aged 13–15years.
Materials and methodsDescriptive study of SARs reported following administration of this vaccine to the PCVC between 2007 and 2011.
ResultsThe clinical symptoms most frequently reported were dizziness, headache, and syncope.
Reporting rates of syncope or loss of consciousness and seizures with qHPV vaccine were 17 and 3.2 per 100,000 doses administered, respectively, and 15 and 1.6 for syncope or loss of consciousness and syncopal seizures occurred on the day of vaccination. The reporting rates of syncope or loss of consciousness and seizures were 6.4 and 0.4, for the other vaccines.
ConclusionsConsistent with the media attention generated, and with results from other studies, the reporting rates of syncope or loss of consciousness and seizures were higher for the HPV vaccine than for other vaccines given in adolescence. Nevertheless, the overall information obtained on SARs following the qHPV vaccine suggests a good safety profile.
En 2009, 2 casos de convulsiones en adolescentes tras la administración de la vacuna tetravalente frente al virus del papiloma humano (VPH) generaron impacto mediático y afectaron negativamente la confianza del público en esta vacuna.
Nuestros objetivos fueron describir las sospechas de reacciones adversas (SRA) notificadas al Centro Autonómico de Farmacovigilancia de la Comunidad Valenciana (CAFCV) tras la administración de la vacuna frente al VPH y comparar la tasa de notificación de síncope y convulsiones de esta vacuna con la de otras vacunas administradas en adolescentes.
Material y métodosEstudio descriptivo de las notificaciones de SRA relacionadas con esta vacuna recibidas por el CAFCV entre 2007 y 2011.
ResultadosLas manifestaciones clínicas más comunicadas fueron mareos, cefalea y síncope.
Las tasas de notificación de síncope o pérdida de conciencia y convulsiones con la vacuna frente al VPH fueron de 17 y 3,2 por 100.000 dosis administradas, respectivamente, y de 15 y 1,6 para síncope o pérdida de conciencia y convulsiones sincopales ocurridas el día de la vacunación. Las tasas de notificación de síncope o pérdida de conciencia y convulsiones fueron de 6,4 y 0,4 para otras vacunas.
ConclusionesLas tasas de notificación de síncope o pérdida de conciencia y convulsiones fueron mayores para la vacuna frente al VPH que para otras vacunas administradas en adolescentes; esto es consistente con la atención mediática originada por la vacuna y con hallazgos de estudios previos. No obstante, la información obtenida sobre las SRA a la vacuna sugiere un buen perfil de seguridad.
The vaccine against human papillomavirus (HPV) is indicated for the prevention of cervical cancer and precancerous lesions of the female genital tract.1 There are 2 approved vaccines: Cervarix®, a bivalent vaccine that covers HPV types 16 and 18, and Gardasil®, a tetravalent vaccine that covers HPV types 6, 11, 16 and 18.2 Prior to the approval of both vaccines, clinical trials demonstrated their safety and efficacy in the prevention of precancerous lesions. The adverse effects reported most frequently were pain, redness, and swelling at the site of injection, headache, myalgia, and fatigue.3–6 After the administration of approximately 56 million doses, the Vaccine Adverse Events Reporting System (VAERS), the passive surveillance system of the United States, received a total of 21,194 reports in relation to the quadrivalent HPV vaccine, and the most commonly reported adverse events were syncope, dizziness, nausea, headache, fever, and urticaria, injection-site reactions (pain, redness, and swelling).7 The administration of the quadrivalent vaccine has also been associated to mass psychogenic response in adolescents,8 as happens with other vaccines,9 with symptoms including dizziness, syncope, and neurological complaints without an identified organic cause.
In the case of the bivalent preparation, after the administration of 558,226 doses, 647 adverse events were reported to the National Institute for Public Health and the Environment (RIVM) in the Netherlands, and the adverse events reported most frequently were local reactions, general skin symptoms, syncope, and presyncope.10
Both vaccines were introduced in the Spanish market in September 2007.11 The vaccine against HPV was included in the routine immunisation schedule of the Autonomous Community of Valencia in October 2008 to be administered in schools or healthcare centres to girls aged 14 years.12 In February 2009, a lot of media coverage was given to reports of seizures in 2 girls following the administration of the second dose of the quadrivalent vaccine in close geographical and temporal proximity.13 As an immediate precaution, the Spanish authorities suspended vaccination with the batch concerned in the seizure cases, and the Valencian Community restricted the administration of HPV vaccines to healthcare centres.14,15 Both the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency of Drugs and Health Products [AEMPS]) and the European Medicines Agency (EMA) assessed both cases and determined that they had not been caused by the vaccine.15,16 Furthermore, the EMA concluded that the benefits of the vaccine continued to outweigh its risks, and recommended reinforcing the product information on the possibility of syncope, sometimes accompanied by tonic–clonic movements resembling seizures, as a side effect of vaccination with Gardasil.16 On the other hand, a study conducted by the Center for Disease Control (CDC) of the United States determined that there was no increase in the risk of syncope following administration of the quadrivalent HPV compared with other vaccines administered during adolescence.17
Despite the educational efforts of healthcare authorities, vaccination coverage continues to be lower than expected. Coverage rates of HPV vaccination coverage were 65% in Spain and 58% in the Autonomous Community of Valencia,18 although the rates have recovered in part in the cohort of girls born between 1995, 1996, and 1997 and vaccinated in 2009, 2010, and 2011.14
Passive surveillance in Spain is carried out through the programme for the spontaneous reporting of suspected adverse reactions (SARs) to medicines of the Sistema Español de Farmacovigilancia (Spanish Pharmacovigilance System [SEFV]). Healthcare professionals report suspected events by means of a form known as the “yellow card.” Also, the Autonomous Community of Valencia has a Registro Nominal de Vacunas (Nominal Vaccination Registry [RVN]),19 a system that records the vaccinations given in all public and some private facilities, which also allows electronic reporting of SARs to vaccines to the Centro Autonómico de Farmacovigilancia de la Comunidad Valenciana (Pharmacovigilance Centre of the Valencian Community [CAFCV]).
The aim of this study was to describe the SARs to the HPV vaccine reported to the CAFCV, and to compare the reporting rates for syncope and seizures linked to this vaccine with the reporting rates for the rest of the vaccines administered to girls 13–15 years of age.
Materials and methodsWe conducted a descriptive retrospective study by analysing the SARs to the HPV vaccine reported by healthcare professionals to the CAFCV between September 2007 and December 2011.
The study included reports concerning girls aged 13–15 years that had the minimum information required for processing, as determined by the SEFV criteria.20
The reporting rates for syncope, presyncope, and seizures for the HPV vaccine and the rest of the vaccines administered in girls 13–15 years of age (meningococcal C conjugate vaccine, conjugate meningococcal C, tetanus toxoid, varicella, and tetanus diphtheria [Td] vaccines) calculated by dividing the number of reports for each clinical manifestation by the number of administered doses registered in the RVN; we calculated the 95% confidence intervals (95% CI) by the Clopper–Pearson method.
After grouping the SARs by clinical manifestation, we performed a descriptive analysis of the variables using SPSS version 19.0.
ResultsThe CAFCV received a total of 200 SRA reports. All reports concerned the quadrivalent HPV vaccine, which was the preparation administered in the Valencian Community in the period under study. The study only included 194 of the reports, as the remaining 6 (3%) did not include the date the vaccine was administered, a datum needed to establish a temporal relationship with the clinical manifestations reported. The records of the RVN indicate that 187,385 doses of the vaccine were administered in the same time period, so these 194 reports correspond to a reporting rate of 103 reports (95% CI, 89–118) per 100,000 administered doses.
Description of the analysed reportsOf the 194 reports included in the study, 4 (2%) corresponded to girls aged 13 years, 158 (81%) to girls aged 14 years, and 32 (16.5%) to girls aged 15 years.
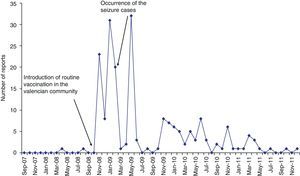
The number of reports was 33 (17%) in 2008, 106 (55%) in 2009, 42 (21.6%) in 2010, and 13 (6.7%) in 2011. There were no reports of SARs in 2007 (September–December). The number of reports per month increased after the introduction of routine vaccination, from none in October 2008 to 23 the following month. The other 2 peaks in reporting coincided with the administration of the second and third doses to the first cohort of vaccinated girls (Fig. 1).
Of all reports, 149 (77%) involved systemic clinical manifestations alone, and 22 (11%) local reactions. The remaining 23 (12%) reported both systemic and local manifestations. The staff of the CAFCV classified 32% of the reports as serious based on the criteria set by the SEFV.20
The 194 reports included 433 SRAs, with an average of 2.2 clinical manifestations by report, and a range of 1–14. The adverse events reported most frequently were dizziness (n=52; 27%), headache (n=44; 23%), syncope or loss of consciousness (n=38; 20%), and fever (n=32; 17%). Reactions reported less frequently included pain at the injection site (n=19; 10%), nausea (n=15; 8%), vomiting, pallor, general malaise (n=12, n=11, and n=11, respectively; 6%), somnolence (n=10; 5%), presyncope (n=8; 4%), and seizures (n=6; 3%).
Syncope or loss of consciousness was described in 38 of the reports (20%). It was accompanied by seizures in 6 cases, which we described separately for that reason. Of the remaining 32 reports, 14 were of syncope or loss of consciousness alone, and 18 of syncope or loss of consciousness in combination with other clinical manifestations, the most frequent of which were dizziness,9 pallor,5 hypotension,4 and vomiting.4 One report also informed of syncope combined with a concussion, and another of syncope in combination with muscle spasms. The episodes of syncope took place on the day of vaccination in 28 out of the 32 reports; in the remaining 4 the latency period lasted between 2 and 10 days, with the episode occurring at a median of 2.5 days postvaccination. The reporting rate for syncope or loss of consciousness was 17 (95% CI, 11.1–23) per 100,000 administered doses, and the rate for syncope occurring on the day of vaccination was 15 (95% CI, 9.4–20.4) per 100,000 administered doses.
Presyncope was described in 8 reports (4%). In 2 of them it was reported by itself and in another 2 along with syncope. In the remaining 4, it was reported with pain in 1, with dizziness in another, and with general malaise in 2. In all 6 reported cases of presyncope (having excluded those in which syncope was reported simultaneously), the onset occurred on the day of vaccination. The reporting rate for presyncope was 3.2 (95% CI, 0.6–5.7) per 100,000 administered doses.
Seizures were described in 6 reports (3%). They were associated to syncope in 4, of which 2 described syncope and seizures (specified as grand mal seizure in one case) as the only two clinical manifestations. Another report described seizures in combination with muscle stiffness, and the last one in combination with motor disturbances (myoclonus and hypertonia). The onset of seizures occurred on the day of vaccination in 5 of the 6 reports, and only occurred later in 1, corresponding to the grand mal seizure, at 50 days postvaccination. The reporting rates per 100,000 administered doses were 3.2 for seizures (95% CI, 0.6–5.7), 2.1 for syncopal seizure (95% CI, 0.04–4.2), and 1.6 for syncopal seizure on the day of vaccination (95% CI, −0.2 to 3.4).
In the period under study, a total of 248,677 doses of vaccines other than the HPV vaccine were administered to girls aged 13–15 years in the Valencian Community, giving rise to 27 reports. Of these, 16 described syncope or loss of consciousness; 6, presyncope; and 1, seizures. The reporting rates for these clinical manifestations were 6.4 (95% CI, 3.2–9.5), 2.4 (95% CI, 0.4–4.3), and 0.4 (95% CI, −0.3 to 1.1) reports per 100,000 administered doses, respectively (Table 1).
Reporting rates observed in the Valencian Community for the HPV vaccine, reporting rates for the HPV vaccine published in other post-licensure studies, and reporting rates for other vaccines observed in the Valencian Community.
| Clinical manifestation | Observed reporting rate: quadrivalent HPV vaccinea (95% CI) | Expected reporting rate: other HPV vaccine studiesb | Observed reporting rate: other vaccinesa (95% CI) |
| Syncope or loss of consciousness | 17 (11.1–23) | 7.827/8.221 | 6.4 (3.2–9.5) |
| Syncope or loss of consciousness on vaccination day | 15 (9.4–20.4) | – | – |
| Presyncope | 3.2c (0.6–5.7) | – | 2.4 (0.4–4.3) |
| Seizures | 3.2 (0.6–5.7) | 0.3d,21 | 0.4 (−0.3 to 1.1) |
| Syncopal seizures | 2.1 (0.04–4.2) | – | – |
| Syncopal seizures on vaccination day | 1.6 (−0.2 to 3.4) | 2.627 | – |
| Total | 103 (89–118) | 53.921/116e,10 | 10.8 (6.7–14.9) |
HPV: vaccine against human papillomavirus.
The clinical manifestations reported to the CAFCV are consistent with those described in the summary of product characteristics4 and those reported by passive surveillance systems following licensure.10,21
The reporting rate of SARs linked to the quadrivalent HPV vaccine in the Valencian Community is higher than the one reported by VAERS in the United States for the same vaccine21 (Table 1). Researchers in the Netherlands considered that the media controversy surrounding the vaccine had an effect on the reporting rate.10 The heavy media coverage of the introduction of the vaccine in the routine vaccination schedule and the seizure episodes suspected to be linked to the vaccine in 2009 may have led to an increased reporting rate in the Valencian Community, too. This is a known effect that is manifested when a pharmacological agent is subject to media exposure, be it because there is a well-known association to a reaction, or because regulatory authorities have issued a warning.22 Still, we must take into account that for the period under study, reports with the CAFCV were only filed by healthcare professionals, while in the VAERS and RIVM systems anyone can make a report.10,23 The RIVM also uses an actively stimulated surveillance system.24
Furthermore, the reporting rate of SARs linked to the quadrivalent HPV vaccine was greater than the rate observed for vaccines that have been administered to adolescents in the Valencian Community for years (Table 1). This could be explained by the Weber effect, which describes an increased number of reports to passive surveillance systems in the early years post-licensure.25,26 This hypothesis would be supported by the findings represented in Fig. 1, which shows a larger number of reports coinciding with the administration of the 3 doses of the vaccine the first year it was included in the routine schedule,14 with a decline in the second year.
The reporting rates for events linked to the HPV vaccine or other vaccines were calculated without consideration of the issue of polyvaccination, so the rates attributable to the HPV vaccine could be lower than the ones obtained. Furthermore, the reporting rates described in other studies21,27 were not calculated for a specific age group, as we did, and they used the number of distributed doses in the calculations, rather than the number of administered doses which we used.
Syncope or loss of consciousness were the clinical manifestations reported most often to the CAFCV, consistent with the reports to the VAERS and RIVM.10,21 However, the reporting rate both for syncope or loss of consciousness on the day of vaccination and for the total cases of syncope or loss of consciousness was greater than the rates found by other passive surveillance systems,17 and greater than the rates for other vaccines administered to adolescents in the Valencian Community (Table 1). This could also be accounted for by the effects previously described; intense media coverage was followed by the healthcare authorities suggesting the reinforcement of information pertaining to administration of this vaccine and the onset of syncope, at times accompanied by tonic-clonic movements similar to seizures, which may have stimulated a higher number of reports of these clinical manifestations. Another factor to consider is that syncope secondary to vaccination tends to happen within 15min following administration of the vaccine.28 However, since we did not have detailed information on the time elapsed, our calculation of the rates of syncope or loss of consciousness included all cases that occurred on the day of vaccination, so we may have overestimated them.
Concussion was reported along with syncope only once. Other studies have shown greater reporting rates for injuries due to the falls that followed the syncope.21,28 This difference could be explained by the underreporting inherent in passive surveillance systems, although injuries resulting from syncope tend to be considered serious and should consequently be reported. It could also be explained by adequate management of the postvaccination period. Following the convulsion cases reported in 2009, administration of this vaccine was restricted to healthcare centres in the Valencian Community, and these types of events are more likely to be prevented in those settings.
The reporting rate for presyncope linked to the HPV vaccine was greater than the rate associated with other vaccines administered to adolescents, but we did not find studies assessing the reporting rates for this clinical manifestation. This may be due to nonreporting of mild clinical manifestations, especially for pharmacological agents that have not been licensed recently.26
The reporting rate for seizures was higher than that of the VAERS21 and higher than the reporting rate for other vaccines administered in adolescence (Table 1). This, too, could be explained by the impact of the media coverage of the seizures suspected to be linked to the vaccine in Valencia, and by the Weber effect. Since we did not have access to medical histories, all reports that informed of both seizures and syncope were classified as syncopal seizures. The reporting rate for syncopal seizure on the day of vaccination thus calculated was lower than the one published in a previous study,27 although the latter was based on a stimulated reporting system.
Pharmacovigilance uses a variety of quantitative methods to evaluate the association between pharmacological agents and their possible adverse effects based on searching the databases for statistical disproportions to determine whether a reaction is reported more frequently for one agent than for others. One such method is the reported odds-ratio (ROR), which consists of a study of cases and controls in which the controls, referred to as non-cases, are reported adverse reactions other than the one under study.29 Calculating the ROR for syncope, presyncope, and convulsions was not useful in this study because the number of non-cases was very small, with most such clinical manifestations reported in girls aged 13–15 years being linked to vaccination against HPV (27 reports for other vaccines vs 194 reports for the HPV vaccine).
There are limitations to this study. One is that in any system of passive surveillance, there is a higher reporting rate for newly approved agents (Weber effect), and a tendency to underreport well-known adverse reactions for agents that have been licensed for longer periods of time. On the other hand, these systems are very sensitive to the effects of media attention. Also, missing data in the reports and the unavailability of medical histories make reported events difficult to confirm. A temporal association between the administration of a given agent and a reported adverse reaction is not enough to establish causality.26
Nevertheless, our results confirm that none of the reported clinical manifestations diverged from those described in the summary of product characteristics of the vaccine and those reported in another passive surveillance system.4,21 They also provide information about the convulsions and syncope that have been the subject of public debate, suggesting a potential correlation between media attention and reporting rates.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rodríguez-Galán MA, Pérez-Vilar S, Díez-Domingo J, Tuells J, Gomar-Fayos J, Morales-Olivas F, et al. Notificación de reacciones adversas a la vacuna frente al virus del papiloma humano en la Comunidad Valenciana (2007–2011). An Pediatr (Barc). 2014;81:303–309.