We do not have population data in Spain on the application of therapeutic hypothermia (TH). The objective was to examine adherence to management standards during TH of infants with hypoxic-ischemic encephalopathy (HIE).
MethodMulticenter observational cohort study from the beginning of TH (year 2010) in 5 hospitals in a Spanish region, until year 2019.
Results133 patients were recruited, 72% diagnosed with moderate HIE and the rest of them with severe HIE. In 84% of infants, passive hypothermia was started at birth. Active TH was started at a median age of 5 h of life (IQR 3.3; 6.3), although the central targeted temperature (33−34 °C) was reached at a median age of 3.5 h (IQR 1; 6). Those born extramural, initiated active TH 3.3 h on average later than those born intramural, but without differences in the age at which the targeted temperature was reached.
Sedoanalgesia was used in 97%. 100% were monitored with amplitude-integrated EEG and 59% with cerebral oxymetry. MRI was performed in 94% with moderate HIE vs. 65% with severe; P < .001. Neuron-specific enolase in cerebrospinal fluid was determined in 42%. The average duration of rewarming was median 10 h (IQR 8; 12), with no differences depending on the degree of HIE (P = .57).
ConclusionsThe implementation of TH successfully met the standards. However, aspects of care that could be improved were detected. Auditing newborn care with HIE is crucial to achieving programs with a high quality of care in each region
No disponemos de datos poblacionales en España sobre la aplicación de la hipotermia terapéutica (HT). El objetivo fue examinar la adherencia a los estándares de manejo durante la HT de los recién nacidos (RN) con encefalopatía hipóxico-isquémica (EHI).
MétodoEstudio observacional de cohortes multicéntrico desde el inicio de la HT (2010) en una región extensa española, hasta el año 2019.
ResultadosSe incluyeron 133 pacientes, el 72% con EHI moderada y el resto con EHI grave. En el 84% se inició hipotermia pasiva en paritorio. La HT activa comenzó a las 5 horas de vida (RIC 3,3 a 6,3), si bien, la temperatura diana central (33−34 °C) se alcanzó a una edad de 3,5 horas (1;6). Los nacidos extramuros iniciaron la HT activa 3,3 horas de media más tarde que los intramuros, pero sin diferencias en la edad a la que se alcanzó la temperatura diana.
El 96% recibió sedoanalgesia. El 100% fue monitorizado con electroencefalografía integrada por amplitud y el 59% con oximetría cerebral. La RM se realizó en el 94% con EHI moderada vs el 65% con grave; P < .001. Se determinó enolasa neuronal-específica en LCR en el 42% de los pacientes. La duración media del recalentamiento fue 10 horas (RIC 8 a 12), sin diferencias según el grado de EHI (P = ,57).
ConclusionesLa aplicación de la HT cumplió satisfactoriamente con los estándares. No obstante se detectaron aspectos de la atención mejorables. Auditar la atención al recién nacido con EHI es crucial para conseguir programas con una alta calidad asistencial en cada región.
In the last decade, numerous paediatric societies have developed guidelines and recommendations to guide the implementation of therapeutic hypothermia (TH) in newborns with hypoxic-ischaemic encephalopathy (HIE).1–4 Yet, their application in Europe has been heterogeneous,5–9 and differences are found even between the regions of a single country.5,10,11
Since the Sociedad Española de Neonatología (Spanish Society of Neonatology, SENeo) published its guidelines to facilitate the clinical application of TH in 2011,12 the number of facilities in Spain that offers it has been growing, although one study warned that their application differed between regions and that there were opportunities for improvement.11,13 However, unlike other countries,5–7,10 no population-based data have been published in Spain analysing the implementation of this treatment to audit the quality of care provided in TH programmes.14
We conducted a multicentre observational cohort study with the aim of assessing (1) the adherence to standards of care for newborns with HIE during treatment with TH from its introduction in our region in 2010 through 2019, and (2) differences in the implementation of TH based on the severity and origin of patient (inborn vs outborn).
MethodsThe sample of this multicentre observational cohort study was obtained by consecutive enrolment of newborns with a diagnosis of HIE from July 2010 to August 2019 in the 5 hospitals with level III neonatal units that provide TH in and manage patients from the autonomous communities (ACs) of Castilla y León (94 226 km2; 2.408 million inhabitants) and La Rioja (5045 km2; 315 675 inhabitants). These 5 hospitals use servo-controlled whole-body cooling systems: CritiCool® System (MTRE, Yavne, Israel) in 4 centres and Tecotherm Neo (Inspiration Healthcare Ltd) in 1. Two hospitals introduced TH in July 2010 (Hospital Universitario de Burgos [HUBU] and Universitario Río Hortega de Valladolid), one in 2012 (Hospital Clínico Universitario de Valladolid), one in 2014 (Complejo Asistencial Universitario de León [CAULE]) and one in 2015 (Hospital Clínico Universitario de Salamanca).
All hospitals had similar management protocols in adherence with the national recommendations of the SENeo12; and 4 of the 5 participated in a multicentre programme aimed at identifying and monitoring newborns with HIE in the first hours post birth.15,16
The severity of encephalopathy was graded within 6 h of birth using the scale developed by García-Alix et al. in 4 hospitals and the scale developed by Sarnat in the remaining hospital.17,18 We defined moderate to severe neonatal HIE as decreased level of consciousness (lethargy, stupor or coma) following a delivery with factors suggestive of perinatal hypoxia-ischaemia. The latter was defined as at least one of the following: (1) cord blood pH at birth or at 1 h post birth of 7.00 or less; (2) 5-min Apgar score of 5 or less and/or (3) need of resuscitation with intermittent positive pressure for more than 5 min, intubation with or without cardiac massage.
The transport of newborns with HIE was carried out by nonspecialised paediatric transport teams comprising 1 physician, 1 nurse and 1 technician.
Some of the patient data were collected prospectively in the framework of the early identification programme mentioned above. The rest were collected retrospectively through the review of health records, including data on: age at initiation, duration and complications of TH, neuromonitoring and neuroimaging tests, use of sedation and analgesia, respiratory support, nutrition, use of antibiotherapy, multiple organ involvement and neonatal outcome.
We classified amplitude-integrated electroencephalogram (aEEG) recordings based on tracing patterns.19
Statistical analysisWe have expressed qualitative data as percentage distributions and quantitative data using measures of central tendency and dispersion. We compared groups with the χ2 and Student t test or the applicable nonparametric test if the data did not fit a normal distribution (Wilcoxon or Mann-Whitney U test). Statistical significance was defined as a p-value of less than 0.05. The statistical analysis was performed with the software SPSS® Statistics version 20.
The study was approved by the research ethics committee of the coordinating hospital (CEIC 2298).
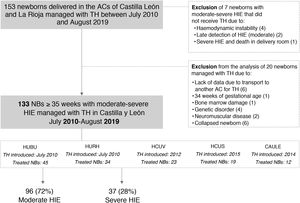
ResultsIn the period under study, 153 newborns received TH; 7 newborns were excluded because they did not. In the analysis, we excluded another 14 newborns that received TH in the context of a different disease, and 6 newborns that received TH in other ACs (Fig. 1).
Flowchart of patient inclusion and exclusion from the study.
AC, autonomous community; CAULE, Complejo Asistencial Universitario de León; HCUS, Hospital Clínico Universitario de Salamanca; HCUV, Hospital Clínico Universitario de Valladolid; HIE, hypoxic-ischaemic encephalopathy; HUBU, Hospital Universitario de Burgos; HURH, Hospital Universitario Río Hortega de Valladolid; NB, newborn; TH, therapeutic hypothermia.
Of the 133 newborns in the final sample, 96 (72%) received a diagnosis of moderate HIE and 37 (28%) of severe HIE. Those with severe HIE had less favourable perinatal characteristics and a higher frequency of seizures, aEEG abnormalities and multiple organ involvement (P < .001). Of the 28 newborns that died, 22 (79%) had severe HIE, and of these 22, 86% died following withdrawal or withholding of life-sustaining treatment (Tables 1 and 2).
Perinatal characteristics of the cohort.
| Total, N = 133 | Moderate HIE, N = 96 | Severe HIE, N = 37 | P | |
|---|---|---|---|---|
| Outborn | 57/133 (43%) | 37/96 (39%) | 20/37 (54%) | .105 |
| Male sex | 81/133 (61%) | 62/96 (65%) | 19/37 (51%) | .161 |
| Gestational age, weeks | 39 (37−40) | 39 (37−40) | 39 (37−40) | .467 |
| Birth weight, g | 3117 (2675−3460) | 3200 (2700−3500) | 3030 (2500−3275) | .036 |
| Uncomplicated delivery | 18/133 (14%) | 16/96 (17%) | 2/37 (5%) | .089 |
| Cephalic presentation | 118/125 (94%) | 87/92 (95%) | 31/33 (94%) | 1.000 |
| Sentinel events | 58/131 (44%) | 39/94 (41%) | 19/37 (51%) | .306 |
| 1-min Apgara | 2 (1−3) | 2 (1−3) | 0 (0−1) | .000 |
| 5-min Apgarb | 4 (2−6) | 5 (3−6) | 2 (0−4) | .000 |
| 10-min Apgarc | 5 (4−7) | 6 (5−8) | 3 (2−4) | .000 |
| Cord blood pHd | 6.97 (6.80−7.18) | 7.02 (6.87−7.20) | 6.8 (6.79−6.99) | .001 |
| Advanced resuscitation | 102/133 (77%) | 67/96 (70%) | 35/37 (95%) | .002 |
Categorical variables expressed as n/N (%) and continuous variables as median (interquartile range).
Advanced resuscitation: intubation and/or cardiac massage and/or drugs.
HIE, hypoxic-ischaemic encephalopathy.
Data not available for 1a, 1b, 27c and 31d patients.
Characteristics of neurologic and systemic involvement in the cohort.
| Total, N = 133 | Moderate HIE, N = 96 | Severe HIE, N = 37 | P | |
|---|---|---|---|---|
| Abnormal aEEG tracinga | 116/133 (87%) | 79/96 (82%) | 37/37 (100%) | .000 |
| Pre-hypothermia | 82/120 (68%) | 49/87 (56%) | 33/33 (100%) | .000 |
| During TH | 114/133 (96%) | 78/96 (81%) | 36/37 (97%) | .000 |
| Clinical and/or electrical seizures | 50/133 (38%) | 22/96 (23%) | 28/37 (76%) | .000 |
| Pre-hypothermia | 29/133 (22%) | 14/96 (15%) | 15/37 (41%) | .001 |
| During TH | 38/133 (29%) | 16/96 (17%) | 22/37 (59%) | .000 |
| Pulmonary involvement | 81/133 (61%) | 52/96 (54%) | 29/37 (78%) | .010 |
| Pulmonary hypertension managed with nitric oxide | 18/133 (14%) | 12/96 (13%) | 6/37 (16%) | .575 |
| Mechanical ventilation | 126/133 (95%) | 89/96 (93%) | 37/37 (100%) | .190 |
| Use of inotropes | 100/133 (75%) | 66/96 (69%) | 34/37 (92%) | .006 |
| Renal involvement | 20/133 (15%) | 10/96 (10%) | 10/37 (27%) | .016 |
| Hepatic involvement | 87/133 (65%) | 58/96 (60%) | 29/37 (78%) | .051 |
| Coagulopathy | 61/133 (46%) | 32/96 (33%) | 29/37 (78%) | .000 |
| Thrombocytopenia | 27/133 (20%) | 17/96 (18%) | 10/37 (27%) | .231 |
| Gastrointestinal involvement | 16/133 (12%) | 8/96 (8%) | 8/37 (22%) | .070 |
| Sepsis | 6/133 (5%) | 5/96 (5%) | 1/37 (3%) | 1.000 |
| Death | 28/133 (21%) | 6/96 (6%) | 22/37 (59%) | .000 |
| Age at death, h | 38 (20.5−87.5) | 61 (24−227) | 34 (19−85.75) | .372 |
| Withholding of life-sustaining treatment | 20/27 (74%) | 2/6 (33%) | 18/21 (86%) | .024 |
Categorical variables expressed as n/N (%) and continuous variables as median (interquartile range). Definitions: Pulmonary involvement: need of supplemental oxygen and/or pulmonary hypertension and/or use of nitric oxide. Renal involvement: creatinine > 1.5 mg/dL. Hepatic involvement: hypertransaminasaemia with alanine aminotransferase and/or aspartate aminotransferase > 100 U/L. Gastrointestinal involvement: haemorrhage and/or necrotising enterocolitis. Coagulopathy: abnormal coagulation parameters requiring transfusion of fresh frozen plasma and/or clotting factors. Thrombocytopenia: low platelet count requiring transfusion of platelets. Sepsis: infection with positive blood culture.
HIE, hypoxic-ischaemic encephalopathy.
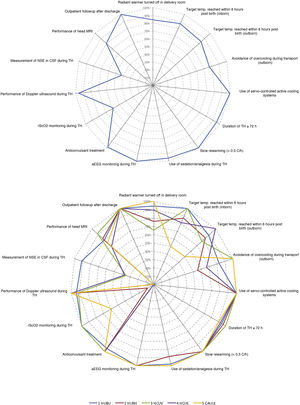
Fig. 3 summarizes the adherence to the main aspects of management documented in our study based on the recommendations of the SENeo guideline. We also took into account 2 aspects that were not considered in the guideline: monitoring of regional cerebral oxygen saturation (rScO2) and measurement of neuron-specific enolase (NSE) levels in cerebrospinal fluid (CSF).
Use of therapeutic hypothermiaIn 84% of the newborns, the radiant warmer was turned off in the delivery room. Servo-controlled whole-body hypothermia (active cooling) was initiated a median of 5 h post birth (interquartile range [IQR], 3.3−6.3), with a median of 4 h (IQR, 1−6) in those with severe HIE vs 5 h (IQR, 4−7) in newborns with moderate HIE (P = .018). However, the target central temperature (33−34 °C) was achieved at age 3.5 h (IQR, 1−6), as 72% of newborns with severe HIE and 56% of newborns with moderate HIE achieved the target temperature with the initial passive cooling measures before initiation of active cooling (Table 3).
Variables associated with the implementation of therapeutic hypothermia.
| Total, N = 133 | Moderate HIE, N = 96 | Severe HIE, N = 37 | P | |
|---|---|---|---|---|
| Radiant warmer turned off in delivery room | 97/115 (84%) | 70/82 (85%) | 27/33 (82%) | .636 |
| Achievement of target temperature (33−34 °C) with passive cooling | 79/131 (60%) | 53/95 (56%) | 26/36 (72%) | .086 |
| Time of initiation of active cooling, hpb | 5 (3.3−6.3) | 5 (4−7) | 4 (1−6) | .018 |
| Duration of rewarming, h | 10 (8−12) | 9.5 (8−12) | 10 (8−10.5) | .567 |
| Temperature on arrival to receiving hospital (outborn), °C | 33 (33−34.5) | 34 (33−34.6) | 33 (32.8−34) | .036 |
| Modification of target temperature | 27/133 (20%) | 14/96 (15%) | 13/37 (35%) | .008 |
| 2/2 pulmonary hypertension | 5/133 (4%) | 5/96 (5%) | 0/37 (0%) | |
| 2/2 refractory coagulopathy | 4/133 (3%) | 3/96 (3%) | 1/37 (3%) | |
| 2/2 haemodynamic shock | 6/133 (5%) | 3/96 (3%) | 3/37 (8%) | |
| 2/2 more than 1 of the above | 12/133 (9%) | 3/96 (3%) | 9/37 (24%) | |
| Discontinuation of active cooling before 72 hpb | 8/133 (6%) | 7/96 (7%) | 1/37 (3%) | .442 |
| Sedation | 128/133 (96%) | 95/96 (99%) | 33/37 (89%) | .021 |
| Boluses | 7/133 (5%) | 5/96 (5%) | 2/37 (5%) | |
| Infusion | 62/133 (47%) | 42/96 (44%) | 20/37 (54%) | |
| Infusion and boluses | 59/133 (44%) | 48/96 (50%) | 11/37 (30%) | |
| Pharmacological treatment | ||||
| Fentanyl | 54/133 (41%) | 38/96 (40%) | 16/37(43%) | .368 |
| Morphine | 1/133 (1%) | 1/96 (1%) | 0/37 (0%) | |
| Fentanyl and morphine | 3/133 (2%) | 1/96 (1%) | 2/37 (5%) | |
| Fentanyl and/or morphine + midazolam | 70/133 (53%) | 55/96 (57%) | 15/37 (41%) | |
| Parenteral nutrition | 81/133 (61%) | 64/96 (67%) | 17/37 (46%) | .028 |
| Enteral nutrition | 13/133 (10%) | 12/96 (13%) | 1/37 (3%) | .111 |
| Antibiotic use | 132/133 (99%) | 95/96 (99%) | 37/37 (100%) | 1.000 |
Categorical variables expressed as n/N (%) and continuous variables as median (interquartile range).
HIE, hypoxic-ischaemic encephalopathy; hpb, hours post birth; 2/2, secondary to.
Fifty-seven percent of the patients were delivered in one of the hospitals that provided TH (inborn), and active cooling in these patients was initiated earlier compared to outborn patients at a median of 4 h post birth (IQR, 2–5) versus 6 h (IQR, 5−8) (P < .001). Adjusting for the severity of HIE, active cooling was initiated a mean of 3.3 h later in outborn infants compared to inborn infants (95% confidence interval [CI], 2.5–4.1). However, there were no differences between outborn and inborn infants in the median age at which the target central temperature was achieved: 3 h (IQR, 1–5) vs 4 h (IQR, 1−6) (P = .21).
Thirty-three patients were born off site and required transport. The median central temperature on arrival to the receiving hospital in those with severe HIE was 33 °C (IQR, 32.8−34) vs 34 °C (IQR, 33−34.7) in those with moderate HIE (P = .04). Overcooling (temperature < 33 °C) was more frequent in newborns with severe HIE compared to those with moderate HIE: 6/19 (32%) vs 7/37 (19%) (P = .33).
Duration and complications of treatmentThe TH protocol was completed in 125 (94%) patients, and rewarming lasted a median of 10 h (IQR, 8−12), with no differences based on the severity of HIE (P = .57). In 27 (20%) of the 133 patients, the target temperature had to be raised due to pulmonary hypertension, coagulopathy and/or severe haemodynamic shock, more frequently in patients with severe HIE (P = .008). In this subset, TH could be withdrawn completely in 8 of the 27 newborns before 72 h post birth (Table 3).
Two infants had subcutaneous fat necrosis. Antibiotherapy was used in all but one of the infants, and an aminoglycoside was included in the management of 114 patients (86%). Six patients (5%) had early-onset sepsis with a positive blood culture. During TH, 61% of the newborns received parenteral nutrition, and 10% enteral nutrition (enteral trophic feeding in 11/13).
Use of sedation and analgesiaNinety-six percent of the patients received sedation/analgesia, 47% via infusion. The most frequent sedation regimen consisted of the combination of an opiate with midazolam (53%), followed by fentanyl as monotherapy (41%). Of the 5 infants that did not receive sedation/analgesia, 4 had severe HIE (coma). All patients but one (127/128) required respiratory support, and 121of them (94%) required mechanical ventilation (Table 3).
Diagnostic tests performed to assess neurologic involvementAll patients were monitored with aEEG and 72% with monitoring of rScO2. Magnetic resonance imaging (MRI) was used in 80% of the patients, 94% of those with moderate HIE and 46% of those with severe HIE (P < .001). The median age at which the MRI scan was performed was 11 days (IQR, 8–15). Of the 22 infants with severe HIE who died, only 3 had been evaluated with MRI.
The levels of ENE in CSF were measured in 42% of the newborns during TH, and the results were available within 24 h in only 7 of these 56 patients.
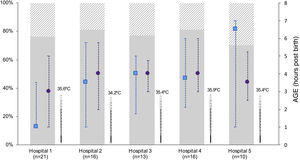
Differences between participating hospitalsThe number of newborns managed with TH varied between hospitals (Appendix B, supplementary tables 1 and 2). While we did not find differences in the age at initiation of active cooling, the median age at which the target central temperature was achieved ranged from 1 h post birth (IQR, 1–4) in the HUBU and 6.5 h post birth (IQR, 1.3–7) in the CAULE (P = .001). In the latter hospital, most patients achieved the target temperature after initiation of active cooling (69%). In contrast, 77% of newborns in the HUBU achieved the target temperature with passive measures before initiation of active cooling (Fig. 2; Appendix B supplementary table 2). When we analysed the diagnostic measures, we found variability between centres in the use of rScO2 monitoring and the measurement of NSE levels (Fig. 3; Appendix B, supplementary table 2).
Characteristics of initiation of hypothermia in inborn patients stratified by hospital.
The figure only shows inborn patients to avoid the bias resulting from the asymmetrical distribution of inborn and outborn patients between hospitals. We present the temperature at admission (median) to the neonatal unit once the newborn was transferred from the delivery room (thermometer symbol), the age at which the target central temperature of 33−34 °C was reached (median, interquartile range) (blue square) and the age at initiation of active cooling (purple circle). The bars represent the distribution of newborns by severity of HIE: moderate (solid grey) and severe (shaded grey). Hospitals: 1 (HUBU), 2 (HURH), 3 (HCUV), 4 (HCUS), 5 (CAULE).
Characteristics of the management of patients treated with hypothermia included in the study based on the recommendations of the SENeo guideline, overall (A) and stratified by hospital (B).
aEEG, amplitude-integrated electroencephalogram; CSF, cerebrospinal fluid; Doppler US, cranial Doppler ultrasound; NSE, neuron-specific enolase; rScO2, regional cerebral oxygen saturation; temp, temperature; TH, therapeutic hypothermia.
The categories “duration of at least 72 h” and “rewarming over a minimum of 8 h” did not include patients who died before 72 h post birth or patients who died during “outpatient follow-up”.
For the first time, we offer population-based data regarding the adherence to care standards in the management with TH of newborns with HIE in Spain. We also offer information that is reported less thoroughly in other case series regarding the characteristics of the newborns based on the severity of HIE.
This multicentre study shows that a high percentage of newborns with moderate to severe HIE are managed with TH, reflecting a very satisfactory accessibility to this treatment in the region. It is likely that these encouraging findings, which show improvement from the 86% reported in a study conducted in Spain that covered the 2012–2013 period, are associated with the existence in our region of a programme aimed at improving the identification of newborns eligible for TH.15,16 we do not know whether similar programmes are being implemented elsewhere in Spain, but their widespread implementation could promote the standardization of the care received by these newborns.14,20
Achieving the target temperature before 6 h post birthA relevant finding of the study was the early time at which the target temperature of 33−34 °C was achieved. The median of 3.5 h post birth is lower compared to the time reported in early clinical trials and other case series in the literature, in which the target temperature was mostly achieved after 4 h post birth.5,7,21–23 Early achievement of the target, repeatedly recommended as a possible strategy to improve the outcomes of TH, is also facilitated by the early HIE detection programme in our region.14,22,24
The initiation of cooling measures in the delivery room was universal and in adherence with the recommendations of the International Liaison Committee on Resuscitation (ILCOR).25 This, combined with the initiation of passive cooling measures in the sending hospital and during transport, explains how outborn patients achieved the target temperature at a similar age as inborn patients, despite active cooling being initiated later at the receiving hospital.23 However, stricter temperature monitoring is needed, as evinced by the fact that 23% of transported newborns were overcooled at arrival to the receiving hospital, despite the existence of a protocol with recommendations for cooling during transport.15 In most regions in Spain, there are deficiencies in the availability of both specialised neonatal transport teams and servo-controlled cooling systems for transport.26
International recommendations, including the guidelines of the SENeo, emphasise the importance of protracted rewarming when a return to normal body temperature is required or at the end of the recommended 72 h of TH.12 Our study showed that rewarming is performed slowly, amply complying with the recommendation of rewarming at a rate of 0.5 °C or less per hour.12,25
Sedation and continuous neuromonitoringAdherence to established care standards was very satisfactory when it came to the use of sedation and neuromonitoring.
Sedation/analgesia was used in practically every patient, and most required mechanical ventilation. Although in a high percentage of patients the need of respiratory support was associated with the underlying disease, and the need of intubation was singled out in a nationwide survey as the drawback of providing sedation to these patients, so that only 70% reported using it routinely.13 One aspect that needs resolving, therefore, is which drug or combination of drugs would be most appropriate based on its pharmacokinetics in the context of TH, with the aim of minimising discomfort while avoiding respiratory depression.27
On the other hand, neuromonitoring was widely used, mainly with aEEG and, to a lesser extent, rScO2 monitoring. Although there are no firm recommendations on the type of monitoring required for adequate management of these patients, in the case of aEEG there is ample evidence of its usefulness in guiding the identification of patients with HIE, detecting epileptic seizures and predicting outcomes in the first 3 days post birth.28,29 The usefulness of rScO2 monitoring in HIE remains to be established, although there is growing evidence that it contributes valuable information on the balance of regional cerebral oxygen availability and utilization and the haemodynamic status and outcomes.30,31
Diagnostic methods to establish the extent of brain damageMagnetic resonance imaging contributes objective information on the severity of brain damage and has a high predictive value.32 Having this information is particularly important in the first days of life, when decisions regarding redirection of curative care to palliative care may be made in the most severely ill patients.33 Although performance of MRI was universal in patients who survived, an MRI scan was only performed in 3 of the 22 patients with severe HIE who died. Since in most of these patients (86%) there was redirection of care, it is clear that the MRI was not routinely included in the decision-making process. This is an opportunity for improvement, and in this context, measurement of biochemical markers of brain damage, such as the levels of NSE in CSF should also be contemplated.34 Both tools are particularly useful in the case of prognostic uncertainty, as they are not affected by sedative drugs, unlike clinical manifestations, the aEEG or the rScO2. Their drawbacks include the need of transport to the radiology room or performance of lumbar puncture in severely ill and unstable patients, in addition to the limitations in many hospitals when it comes to results becoming available within the necessary time window.13
Enteral nutrition, antibiotherapy and family bonding in therapeutic hypothermiaIn our sample, enteral feeding was only used in 10% of the patients, which stood in contrast to other studies.35 However, the current evidence on how to administer it and in which cases is scarce, although the practice appears safe and could help reduce the time elapsed to full oral feeding as well as the length of stay.35
Empirical antibiotherapy was provided routinely to these patients due to their increased risk of early-onset sepsis.36 This approach is controversial compared to the option of monitoring biochemical markers and initiating antibiotherapy once the findings suggest the development of sepsis, but it is important to take into account that biochemical marker levels may change as a result of asphyxia itself.37 On the other hand, the substantial use of aminoglycosides is alarming, as these agents should be used with caution due to the increased risk of toxicity in newborns undergoing TH.38
Only 6% of parents held their babies during treatment. Stress and suffering have been described in the families of newborns managed with TH, as well as anxiety at the thought of approaching the infant and participating in providing care.39 However, when parents provided routine care and comfort to newborns with TH they had positive feelings, including a connection to the child, reinforcement of their parental role and that these practices could be safe.39,40
There are limitations to our study. One of them is its ambidirectional design, which entails uncertainty as regards the precision of the data obtained from health records. In addition, the assessment of the severity of HIE may not have been homogeneous across facilities,16 although we believe that the existence of a programme for the early identification of HIE minimises this potential bias and is one of the strengths of the study.
ConclusionOur study evinced an excellent accessibility of TH in this region of Spain and early achievement of the target temperature. We also found that the application of TH adheres to established standards. Possible areas of improvement in our region are: (a) reducing heterogeneity between hospitals in the age at which the target temperature is achieved; (b) in newborns with severe HIE, performance of MRI and measurement of biomarkers of injury when decisions are being made about redirection of care; (c) reducing the use of aminoglycosides and (d) improving the participation of the family in the care of newborns with HIE during TH. Similar studies evaluating the care of newborns with HIE are crucial in order to identify aspects of care that require improvement and to develop high-quality care programmes in each region.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Ms. Sara Calvo (Fundación Burgos por la Investigación de la Salud) for her help in the statistical analysis.
Inés Esteban. Department of Paediatrics (Neonatology), Hospital San Pedro de Logroño, Logroño, Spain. Electronic mail: iesteban@riojasalud.es
María del Pilar Jiménez. Department of Paediatrics (Neonatology), Complejo Asistencial de Ávila, Avila, Spain. Electronic mail: mjimenezsau@saludcastillayleon.es
Marisa Serrano. Department of Paediatrics, Complejo Asistencial de Soria, Soria, Spain. Electronic mail: apenabu@saludcastillayleon.es
Natalio Hernández. Department of Paediatrics (Neonatology), Complejo Asistencial de Zamora, Zamora, Spain. Electronic mail: nhernandezg@saludcastillayleon.es
Myriam Hortelano. Department of Paediatrics (Neonatology), Complejo Asistencial de Segovia, Segovia, Spain. Electronic mail: mhortelano@saludcastillayleon.es
Maria Teresa Prada. Department of Paediatrics, Hospital El Bierzo, Ponferrada, Spain. Electronic mail: tprada@saludcastillayleon.es
Florentino Barbadillo. Department of Paediatrics, Hospital Santos Reyes, Aranda de Duero, Burgos, Spain. Electronic mail: sschuffelmann@saludcastillayleon.es
Pablo Diego Gayte. Department of Paediatrics, Hospital Santiago Apóstol, Miranda de Ebro, Burgos, Spain. Electronic mail: pdiego@saludcastillayleon.es
Additional information on the members of the ARAHIP Group can be found in Appendix A.
Please cite this article as: Vega-del-Val C, Arnaez J, Caserío S, Gutiérrez EP, Castañón L, Benito M, et al. Adherencia a los estándares en el tratamiento con hipotermia del recién nacido con encefalopatía hipóxico-isquémica. An Pediatr (Barc). 2022;97:30–39.