The main preventive measure against invasive meningococcal disease is vaccination. The aim of our study was to evaluate the acceptability of the meningococcal B (MenB) vaccine and socioeconomic inequalities in the access to the vaccine in the Community of Madrid in the period prior to its introduction in the immunization schedule.
Materials and methodsWe conducted an observational and ecological descriptive study in the cohort of children born between 2016 and 2019 using population-based electronic records. We calculated the vaccination coverage and analysed factors associated with vaccination status, determined the spatial distribution of vaccination coverage and the deprivation index (DI) and assessed the association between them by means of spatial regression.
ResultsWe observed an increasing trend in primary vaccination coverage, from 44% in the cohort born in 2016 to 68% in the 2019 cohort. We found a statistically significant association between vaccination status and the DI (OR of primary vaccination in areas with DI5 compared to areas with DP1, 0.38; 95% confidence interval, 0.39−0.50; P<.001). The spatial analysis showed an inverse correlation between the DI and vaccination coverage.
ConclusionsThe rise in the coverages of the MenB vaccine shows acceptance by the population. The association between socioeconomic level and vaccination coverage confirms the existence of health inequality and underlines the importance including this vaccine in the immunization schedule.
La principal medida de prevención frente a la Enfermedad Meningocócica Invasiva es la vacunación. El objetivo de este estudio es evaluar la aceptabilidad y las desigualdades socioeconómicas en el acceso a la vacuna frente a meningococo B (MenB) en la Comunidad de Madrid en el periodo previo a la introducción de la misma en el calendario.
Materiales y métodosSe realizó un estudio observacional descriptivo en la cohorte de niños/as nacidos entre 2016 y 2019, de tipo ecológico, empleando registros poblacionales electrónicos. Se describieron coberturas de vacunación, se analizaron factores asociados al estado vacunal, se describieron las distribuciones espaciales de cobertura de vacunación y de índice de privación (IP) y se analizó la asociación entre ambas mediante regresión espacial.
ResultadosSe observó una tendencia creciente de las coberturas de primovacunación, pasando de un 44% en la cohorte de nacidos en el año 2016 a un 68% en la cohorte de 2019. Se encontró asociación estadísticamente significativa entre el estado vacunal y el IP (OR de primovacunación en zonas con IP5 respecto a zonas con IP1: 0,38; IC95% 0,39−0,50; P<,001). El análisis espacial mostró correlación inversa entre el IP y la cobertura de vacunación.
ConclusionesEl ascenso de las coberturas de esta vacuna muestra aceptación por parte de la población. La relación entre nivel socioeconómico y cobertura de vacunación confirma la existencia de una desigualdad en salud y subraya la importancia de su inclusión en calendario.
Invasive meningococcal disease is an important public health problem since, while its incidence is fortunately low, it is associated with a high fatality rate (10%) and a high probability of severe long-term sequelae.1–3
The causative agent is Neisseria meningitidis, commonly known as meningococcus, a gram-negative diplococcus whose polysaccharide capsule allows differentiation of up to 13 serogroups, of which 6 cause nearly 100% of cases of invasive meningococcal disease: serogroups A, B, C, W, X and Y.1–3 Among them, serogroup B (MenB) is the one that has caused the most cases in Spain overall and in the Community of Madrid in particular in the past two decades.4,5
Vaccination is the main preventive strategy against this disease available to us.1–3 The lifetime vaccination schedule proposed by the Interterritorial Council of the National Health System (ICNHS) calls for administration of the meningococcal C vaccine, first introduced in 2000, at 4 and 12 months post birth, and administration of the quadrivalent vaccine against serogroups A, C, W and Y, introduced in 2019, at age 12 years, in addition to catch-up vaccination through age 18 years.6 Since 2015,7,8 the Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Pediatrics) recommends vaccination of infants against meningococcal group B disease, and routine vaccination of all infants with meningococcal B vaccine has been included in the publicly funded lifetime vaccination schedule of the national health system.6
The vaccine recently introduced in the vaccination schedule is 4CMenB, a protein-based vaccine that includes 4 subcapsular components.9 It was approved by the European Medicines Agency in 2013 for use from age 2 months.10 In recent years, several studies have been published estimating a high vaccine efficacy and effectiveness in different care settings in the United Kingdom,11 Portugal12 or Canada.13 In 2021, a nationwide case-control study conducted in Spain was published that reported an effectiveness of 73% in individuals who had completed the vaccination series in the past year and 97% in fully vaccinated infants aged less than 1 year.14 The vaccine has proven safe.9 The economic evaluation conducted in relation to its inclusion in the routine childhood vaccination schedule of Spain has been unfavourable to date.15,16 Although previous evaluations carried out by the Working Group of the Advisory Body on Vaccines of the National Health System resulted in the decision not to include this vaccine in the immunization schedule based on the current epidemiological trends,17 the regional governments of some of the autonomous communities, including Canary Islands,18 Castilla y León,19 Andalusia20 and Catalonia,21 decided to include it between years 2019 and 2022. In the last trimester of 2022, in light of the recent scientific evidence on its effectiveness in Spain, the Working Group on MenB of the Advisory Body on Vaccination Programmes and Records carried out a new evaluation regarding the inclusion of this vaccine in the lifetime vaccination schedule of the National Health System, resulting in the approval by the ICNHS of its introduction in the 2023 schedule as a series of 3 doses given at ages 2, 4 and 12 months.16
The availability of the vaccine and the recommendation of its administration by paediatricians (to be paid by the user) in the years prior to its introduction in the routine vaccination schedule of the ICNHS allowed us to assess, on one hand, the acceptability of this vaccine in the population and, on the other, the potential association between vaccination and socioeconomic status in the Community of Madrid during this period. These two factors are important ethical aspects to take into account in the evaluation of potential changes to the immunization schedule22 and, in fact, the findings of the study presented in this article supported the decision to include this vaccine in the schedule. The effectiveness criterion, in addition to other aspects that were discussed in light of the findings of our study, took precedence over economic considerations in this decision.
Thus, the aim of our study was, on one hand, to determine the meningococcal B vaccine coverage in children born between 2016 and 2019 in the Community of Madrid and to assess the association between vaccination coverage and the and the deprivation index (DI) in each basic health zone (BHZ, the smallest administrative division in the public health system) in the period preceding the inclusion of the vaccine in the routine vaccination schedule.
Material and methodsStudy designWe conducted an ecological population-based observational and descriptive study in which the unit in the spatial analysis of the Community of Madrid was the BHZ.23
Study populationThe study included every child born in the 2016–2019 period included in the public health care system card database of the Community of Madrid.
Data sources and study variablesThe sources of data were the vaccination register (SISPAL Vacunas),24 the records database for Community of Madrid health care system users with an active card (CIBELES)25 and cartographic data for the Community of Madrid, including BHZ divisions.
We calculated vaccination coverage estimates by reviewing vaccination records for the period ranging from January 2016 to March 2022, which include the number of doses and dates of administration. We established the following categories: at least 1 dose of meningococcal B vaccine received before age 24 months (MenB1d), at least 2 doses received before age 24 months (MenB2d) and at least 3 doses received before age 24 months (MenB3d). In the analysis of factors associated with vaccination status and the spatial analysis, we only used the MenB2d and MenB3d categories, interpreted as receipt of primary vaccination series and fully vaccinated status, respectively.
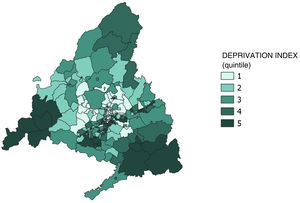
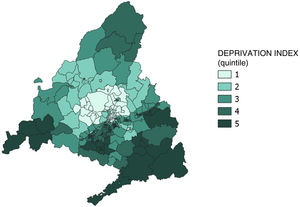
We obtained data on the independent variables of sex, date of birth and country of birth from the health care user card database. We used the DI variable26 as a proxy measure of socioeconomic status. It is a measure of the degree of social and economic deprivation developed by the Sociedad Española de Epidemiología (SEE, Spanish Society of Epidemiology) and calculated using data for a series of indicators retrieved from the 2011 population census, such as the percentages in the population of manual workers, temporary workers, unemployment, low educational attainment (overall) and low educational attainment (in young people) and primary residences without internet access. The DI data of the SEE is divided by census area, and in our study, we calculated the DI for each BHZ as the mean of the DIs of the census areas it contained. This variable can be treated as a continuous quantitative or ordinal variable, if categorised, and its interpretation is ecological. In the present case, we categorised it quintiles, with the first quintile corresponding to the least socioeconomic deprivation and the fifth quintile to the greatest deprivation.
AnalysisAlthough there are 286 BHZ in the Community of Madrid, 19 are new BHZs that do not have any primary care centre within their boundaries, so they have no health care users assigned to them based on their addresses. In the ecological analysis, we imputed the vaccination coverage values of the adjacent BHZ to each of these zones.
We calculated vaccination coverage values as the percentage of children who had received at least 1, 2 or 3 doses of MenB vaccine, so that the numerator corresponded to the number of children born in 2016–2019 with a Community of Madrid public health care user card who had received 1, 2 or 3 doses (MenB1d, MenB2d, MenB3d) of vaccine according to the SISPAL vaccine record database, and the denominator to the total number of children born in 2016–2019 based on the records of the CIBELES population database.
We analysed the factors associated with vaccination status by means of two logistic regression models, calculating the odds ratios for MenB2d status (primary vaccination) or MenB3d status (full vaccination) in relation to the DI (categorised by quintiles) and adjusted for sex and country of origin.
We represented the spatial distribution of the MenB2d and MenB3d vaccine coverage and the DI by BHZ using choropleth maps. We used spatial empirical Bayesian smoothing so that the resulting vaccination coverages and DIs in each BHZ corresponded to the mean of the values of the BHZ and the adjacent BHZs. The purpose was to identify potential spatial patterns.
In addition, we carried out an exploratory spatial autocorrelation analysis of vaccine coverage by BHZ calculating the Moran index (I) and local spatial cluster analysis by means of local indicators of spatial association (LISAs).
We generated scatterplots to analyse the association between vaccination coverage and DI in each BHZ.
Lastly, we fitted 2 spatial lag regression models to measure the magnitude of the association between vaccination coverage and the DI taking spatial location into account. In the first model, the dependent variable was the MenB2d vaccination coverage, and in the second model, it was the MenB3d vaccination coverage.
The data handling and statistical analysis were conducted with the STATA software version 15. The spatial analysis was carried out with the free and open-source QGIS and GeoDa geographic information systems.
Ethical considerationsGiven the characteristics of the study, it was not possible to obtain informed consent from the included subjects. All the data were retrieved from population databases and had been anonymised, and it was handled confidentially and in adherence with current personal data protection regulations and the working protocol of the Directorate General of Public Health; therefore, since the study was conducted within the framework of the use of population data for public health purposes, it did not require evaluation by an ethics committee.
ResultsThe study universe amounted to 232 901 children.
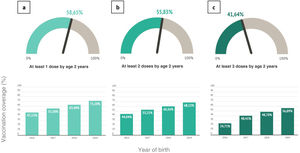
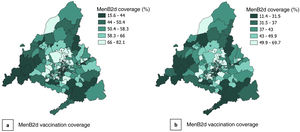
Over the study period, the MenB1d vaccination coverage was 58.65%, increasing over time from 47% of children born in 2016 to 71% of children born in 2019. The overall MenB2d vaccination coverage was 55.83% for the study period, increasing from 44% in the 2016 birth cohort to 68% in the 2019 cohort. The overall MenB3d vaccination coverage was 41.64% overall, increasing from 25% in the 2016 cohort to 56% in the 2019 cohort (Fig. 1).
In the analysis of the factors associated with vaccination status, we found a statistically significant association between vaccination status and DI. The probability of being vaccinated in children who lived in BHZs above the last quintile (DI5) was 62% smaller for the primary series (MenB2d adjusted OR, 0.38; 95% CI, 0.37−0.39; P<.001) and 51% smaller for full vaccination (MenB3d adjusted OR, 0.49; 95% CI, 0.47−0.50; P<.001) compared to children living in BHZs below the first DI quintile (DI1), independently of sex and country of origin. We also found significant differences in the probability of being vaccinated based on the country of origin, with a substantially lower coverage in children of immigrant origin compared to children of Spanish descent. We did not find statistically significant differences in the probability of being vaccinated based on sex (Table 1).
Logistic regression models: analysis of the factors associated with MenB vaccination status.
| MenB2d | MenB3d | |||||||
|---|---|---|---|---|---|---|---|---|
| aOR | P | 95% CI | Observed decrease (%) | aOR | P | 95% CI | Observed decrease (%) | |
| [0.1-9]DI (reference: DI1) | ||||||||
| DI2 | 0.7651 | <.001 | 0.7450−0.7858 | 23.49% | 0.8609 | <.001 | 0.8392−0.8831 | 13.91% |
| DI3 | 0.7090 | <.001 | 0.6915−0.7269 | 29.10% | 0.8386 | <.001 | 0.8188−0.8589 | 16.14% |
| DI4 | 0.4756 | <.001 | 0.4626−0.4890 | 52.44% | 0.5759 | <.001 | 0.5603−0.5921 | 42.41% |
| DI5 | 0.3769 | <.001 | 0.3666−0.3875 | 62.31% | 0.4871 | <.001 | 0.4738−0.5009 | 51.29% |
| Sex (reference: male) | 0.9925 | .386 | 0.9757−1.0096 | ― | 0.9905 | .270 | 0.9739−1.0074 | ― |
| [0.1-9]National origin (reference: Spain) | ||||||||
| North Africa | 0.0178 | <.001 | 0.0113−0.0279 | 98.22% | 0.0198 | <.001 | 0.0112−0.0350 | 98.02% |
| Rest of Africa | 0.0464 | <.001 | 0.0288−0.0747 | 95.36% | 0.0358 | <.001 | 0.0177−0.0722 | 96.42% |
| Central and South America | 0.0330 | <.001 | 0.0292−0.0747 | 96.70% | 0.0272 | <.001 | 0.0228−0.0325 | 97.28% |
| Rest of America | 0.1489 | <.001 | 0.1316−0.1685 | 85.11% | 0.1567 | <.001 | 0.1347−0.1823 | 84.33% |
| Philippines | 0.0664 | <.001 | 0.0286−0.1543 | 93.56% | 0.0388 | <.001 | 0.0095−0.1589 | 96.12% |
| China | 0.2204 | <.001 | 0.1733−0.2804 | 77.96% | 0.2042 | <.001 | 0.1518−0.2747 | 79.58% |
| Rest of Asia | 0.0478 | <.001 | 0.0354−0.0646 | 95.22% | 0.0282 | <.001 | 0.0169−0.0471 | 97.18% |
| Western Europe | 0.1367 | <.001 | 0.1206−0.1593 | 86.33% | 0.1369 | <.001 | 0.1417−0.2303 | 86.31% |
| Romania | 0.1757 | <.001 | 0.1437−0.2148 | 82.43% | 0.1806 | <.001 | 0.1417−0.2303 | 81.94% |
| Rest of Europe | 0.1652 | <.001 | 0.1482−0.1842 | 83.48% | 0.2170 | <.001 | 0.1921−0.2451 | 78.30% |
| Unknown | 0.1008 | .032 | 0.0125−0.8161 | 89.92% | 0.1832 | .111 | 0.0228−1.4753 | ― |
aOR, adjusted odds ratio; CI, confidence interval; DI, deprivation index (DI1, 1st quintile; DI2, 2nd quintile; DI3, 3rd quintile; DI4, 4th quintile; DI5, 5th quintile); MenB2d, (vaccination status) at least 2 doses of meningococcal B vaccine before age 2 years; MenB3d, (vaccination status) at least 3 doses of meningococcal B vaccine before age 2 years.
Figs. 2 and 3 present the spatial distribution of DI and the MenB2d and MenB3d coverage by BHZ. They show significant heterogeneity between BHZs, especially within the city of Madrid, although there is a predominance of a high DI and low vaccination coverage in the southern area of the city in addition to the south and southwest areas of the Community of Madrid.
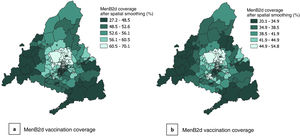
After spatial smoothing (Figs. 4 and 5), the distribution patterns emerge more clearly: low DIs and high vaccination coverage rates can be seen in the northern area of the city of Madrid and the municipalities north of the city, and high DIs and low vaccination coverage rates in the southern area of the city and the periphery of the Community of Madrid, chiefly in the south and south-west.
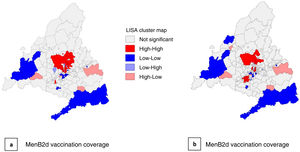
The exploratory spatial analysis showed a moderate spatial autocorrelation in vaccination coverage, both for the MenB2d (Moran I, 0.297) and the MenB3d (Moran I, 0.225). In the LISA cluster analysis, we found 39 BHZs with a high MenB2d vaccination coverage and 24 with a high MenB3d coverage surrounded by other BHZs with high vaccination coverages, chiefly in the northern part of the city of Madrid and municipalities north of the city. There were also 31 BHZs with a low MenB2d coverage and 24 BHZs with a low MenB3d coverage surrounded by other BHZs with low vaccination coverage in the southern part of the city of Madrid in addition to the southern and southwestern areas of the Community of Madrid (Fig. 6).
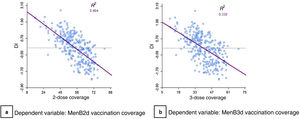
We found a moderate inverse correlation between DI and vaccination coverage within BHZs both for the MenB2d (R2=0.464) as well as the MenB3d (R2=0.33) (Fig. 7).
The spatial regression analysis showed that for each point the DI of a BHZ increased, the MenB2d vaccination coverage decreased by 7.31% (P<.001) and the MenB3d coverage by 5.44% (P<.001), accounting for spatial effects.
DiscussionVaccination against meningococcal disease is an important public health strategy whose implementation must be guaranteed. Our study shows that in the years preceding the introduction of the MenB vaccine in the vaccination schedule of the ICNHS, the vaccination coverage in the paediatric population of the Community of Madrid exceeded the coverage predicted on account of it not being included in the official vaccination schedule, in addition to exhibiting an increasing trend. This reflects not only that the vaccine was acceptable to the population, but that there was an increasing demand for it.
Based on the current evidence, one of the main factors contributing to the acceptability of the vaccine would be the recommendation by paediatricians,27 in addition to parental perceptions regarding the vulnerability of the infant to meningococcal disease.28 Many other aspects may be involved in the acceptability of the vaccine, including demographic, social, cultural and, needless to say, socioeconomic factors.27,28 It is reasonable to expect that the meningococcal B vaccination coverage, which, based on the findings of our study already neared 70% for primary vaccination in the 2019 cohort, will increase considerably after the introduction of the vaccine in the nationwide routine vaccination schedule. In fact, given that some autonomous communities in Spain included this vaccine in their vaccination schedules earlier on, regional data are already available: in Andalusia, where the vaccine was introduced in the 2021 schedule for infants born between October 2021 and March 2022, the primary vaccination coverage for this cohort reached 98%.29 We can also consider the data available for other vaccines. For example, the pneumococcal conjugate vaccine, recommended by paediatricians since it was authorised for distribution in Spain in 2001,30 but not introduced in the routine vaccination schedule of ICNHS until 2015.31 In the years before it was included in the official schedule, the vaccination coverage was low (around 46%),30 but once it was publicly funded, the coverage for primary vaccination reached 95% in the 2016 cohort and 97.7% in the 2017 cohort.32
Our study also contributes evidence of the association between socioeconomic status and vaccination against meningococcal B disease, revealing health inequality in the period preceding the inclusion of the vaccine in the routine schedule. Our findings showed that the probability of having completed the primary vaccination series against MenB was 62% lower in children residing in areas of lower socioeconomic status compared to children in areas of higher socioeconomic status. Studies in the international literature have reported similar findings. A study conducted in Japan in 2018 showed that the probability of vaccination against rotavirus (not publicly funded in the country) was 51% lower in children of low-income families.33 In 2013, Ganczak et al. reported that in Poland, children from families of high socioeconomic status were 3.46 times more likely to receive self-paid vaccines compared to children from families of low socioeconomic status.34
The conditions required to introduce changes to the vaccination schedule were established by the Advisory Body on Vaccination Programmes and Records in 2011. There are 5 criteria that, together, set a framework for the systematic analysis of the arguments in favour and against the implementation and prioritising of changes to the vaccination schedule: burden of disease, vaccine effectiveness and safety, repercussions of introducing the change to the vaccination schedule, ethical considerations and economic evaluation.22
The results of our study support the decision to include the meningococcal B vaccine in the routine lifetime vaccination schedule recommended for 2023 for ethical reasons in light of the potential repercussions of the decision,21 about which there used to be no evidence for the Madrid area.
Among the main limitations of the study, we ought to highlight both the exclusion of children vaccinated through health care systems other than the public health system of Madrid—amounting to 3% of the population, based on data from 202135,36―and the likely under-recording of vaccines not included in the routine schedule at the time of vaccination, due to which we may have underestimated vaccination coverages. On the other hand, since DI values were generated based on indicators from the 2011 census, it is possible that the DI data were outdated. Furthermore, since this was an ecological study, it is important to consider the bias that may result from it. The spatial analysis yielded findings for BHZs as a whole, and it is important not to make inferences regarding individual BHZs based on the resulting data to avoid ecological fallacy. Our study did not analyse how other sociocultural factors (religious, ideological…) may mediate the association between socioeconomic status and vaccination.
Reproducing this study would be useful to monitor inequality and to verify whether public funding of the vaccine increases access to it in the segments of the population with the lowest socioeconomic status, as expected. This would make it possible to assess the impact of the inclusion of this vaccine in the routine schedule in terms of health inequality.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
I thank my three wonderful advisors for their valuable support in the early stages of my public health career.