The complex chronic condition (CCC) is an increasingly prevalent reality in pediatrics. However, having a CCC does not necessarily mean being a complex chronic patient (CCP). From this perspective, we developed an instrument (PedCom Scale) that would facilitate the identification of the CCP.
Material and methodsInitially, general aspects for the classification of patients as CCP were defined. Subsequently, the items of the scale were developed, scoring them from 0.5 to 4 points. We performed a confirmatory factor analysis (CFA) and the internal consistency was studied using alpha-Cronbach. Concordance was evaluated by intra- and inter-observer study. The gold standard was the classification performed by two evaluators after assessing the patient's medical history. The cut-off point for considering the patient as a CCP was established using the ROC curve.
ResultsThe initial version included 43 items with a global content validity index (CVI) of 0.94. A total of 180 patients were included. After the CFA, one item was eliminated, so the final version consists of 42 items with an CVI of 0.95. The alpha-Cronbach value was 0.723. The intraclass correlation coefficient of the test-retest analysis was 0.998 and 0.996 for the inter-observer study. The cut-off point for considering a patient as a CCP was established at 6.5 points, with this results we obtained a sensitivity of 98% and specificity of 94%.
ConclusionsThe PedCom Scale is an easy-to-use tool focused on the identification of the CCP. In our sample, it presented satisfactory levels of internal consistency and adequate levels of intra- and inter-observer agreement, with good sensitivity and specificity for the identification of the CCP.
La condición crónica compleja (CCC) es una realidad cada vez más prevalente en pediatría. Sin embargo, padecer una CCC no supone necesariamente ser un paciente crónico complejo (PCC). Desde esta perspectiva, nos propusimos el desarrollo de un instrumento (Escala PedCom) que facilitase la identificación del PCC.
Material y métodosInicialmente se definieron aspectos generales para la clasificación de un paciente como PCC. Posteriormente se desarrollaron los ítems de la escala puntuándolos de 0,5–4 puntos. Se realizó análisis factorial confirmatorio (AFC) y se estudió la consistencia interna mediante alfa-Cronbach. La concordancia se evaluó mediante estudio intra e inter-observador. El gold standard fue la clasificación realizada por dos evaluadores tras valoración de la historia clínica del paciente. El punto de corte para considerar al paciente como PCC se estableció mediante curva ROC.
ResultadosLa versión inicial incluyó 43 ítems con índice de validez de contenido global (IVC) de 0,94. Para el estudio se incluyeron 180 pacientes. Tras el AFC se eliminó un ítem, por lo que la versión final consta de 42 ítems con IVC de 0.95. El valor alfa-Cronbach fue 0,723. El índice de correlación intraclase del análisis test-retest fue 0,998 y 0,996 para el estudio inter-observador. El punto de corte para considerar a un paciente como PCC se estableció en 6,5 puntos, con el que se obtuvo una sensibilidad del 98% y especificidad del 94%.
ConclusionesLa Escala PedCom es una herramienta de fácil uso enfocada a la identificación del PCC. En nuestra muestra, presentó adecuada consistencia interna y niveles adecuados de concordancia intra e inter-observador; con buenos resultados de sensibilidad y especificidad para la identificación del PCC.
Complex chronic conditions (CCCs) are increasingly prevalent in pediatrics and associated with a high frequency of hospital admission and readmission with more complications during the stay, substantial use of health care resources and high health care costs, due to which patients with CCCs are a priority for the health care system.1–6
Although patients with CCCs have diverse diagnoses and needs, they share certain characteristics, such as fragility, functional limitations, high use of resources or technological dependency. This has led to an attempt of establishing a single definition for CCC in recent years, but, until today, no agreement has been reached on a definition comprehending the full spectrum of these conditions.1,7–9
One of the most widely used definitions at present is the one proposed by Simon et al., according to which, CCC would be defined as the presence of at least one of the following characteristics10:
- -
Significant chronic conditions in ≥2 body systems that can be expected to last at least a year and can be expected to be episodically or continuously debilitating.
- -
A progressive condition that is associated with deteriorating health with a decreased life expectancy in adulthood.
- -
Continuous dependence on technology for at least 6 months.
- -
Progressive or metastatic malignancies that affect life function.
At the same time, in everyday clinical practice we find that the diagnosis of CCCs does not necessarily imply the presence of chronic medical complexity or that the patient should be considered a chronic complex patient (CCP). And while in this regard the term “medically complex patient” (MCP) could be considered equivalent to the term “CCP”, there is also no consensus regarding the definition of the former, or what needs or degree of functional impairment or dependency would be required to consider a patient as medically complex; in fact, the term is often used interchangeably with CCC.5,8,10–15
These difficulties in its definition have motivated the development of tools to facilitate the identification of CCC, but the instruments currently available, such as the Pediatric Complex Chronic Conditions Classification system, the Pediatric Medical Complexity Algorithm or the Clinical Risk Groups systems are mainly based on diagnosis or the presence of technological dependence, and their use is not always intuitive or simple in daily clinical practice. They were also not developed for the purpose of identifying CCPs.9,12,16–19
Another instrument that is not categorical and not based on specific diagnoses is the Point-of-Care Complexity Screening Algorithm to Identify Children With Medical Complexity, which is based on the definition of patient with special health care needs. In this case, the algorithm is focused on the detection of medical complexity in hospitalised patients, but not in the follow-up at the outpatient level, and it includes a very limited range of devices or technological support. It also cannot be used for identification of CCPs nor is it validated in these patients.20
Thus, there are no instruments specifically designed and validated for the identification of CCPs.
In this context, our research group set out to develop a scale to facilitate the identification of CCPs from a non-categorical perspective and independently of patient diagnoses.
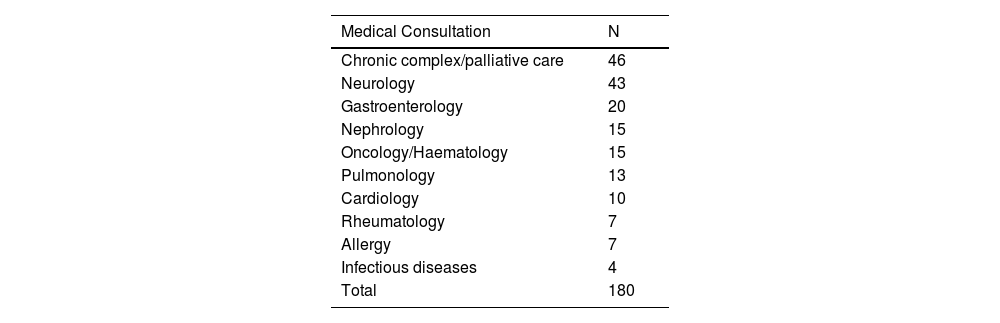
Material and methodsThe study was conducted in a tertiary care hospital in adherence with the ethical standards of the centre, and was approved by the provincial research ethics committee. It included patients managed in the outpatient clinics of the hospital (gastroenterology, neurology, oncology/haematology, rheumatology, cardiology, endocrinology, infectious disease, nephrology, allergy, pulmonology, paediatric complex chronic patient and palliative care) with a duration of follow-up of at least 1 year, excluding those currently experiencing acute decompensation. The study did include patients at the end of life or with irreversible deterioration requiring palliative care.
The development and validation of the scale was organised in 3 phases.
First, the researchers established the general criteria to consider a patient as chronically complex in a consensus process. Thus, classification as a CCP would require meeting certain criteria:
- -
Presence of a disease or clinical condition meeting the definition of CCC proposed by Simon et al.,10 with at least one of the diagnoses of the patient (primary or secondary) included in the classification by Feudtner et al updated in 2014 with international classification of diseases (ICD)-10 codes.16
- -
AND coexistence of more than one condition (≥2) resulting in medical complexity, such as the need of follow-up by multiple specialists, polypharmacy, clinical instability, functional impairment or moderate-to-severe psychomotor delay, need of special treatments, long-term technological support or decreased life expectancy, resulting in impaired wellbeing and quality of life.
- -
Said health care needs must have lasted or should be expected to last at least 12 months, except in the case of technological dependence, in which 6 months suffice.
- -
To avoid exclusion of patients with severe disease and a poor short-term prognosis, such as incurable malignancy, the minimum time requirements do not apply in this kind of situation, taking into account that it is important to avoid the use of the scale when the patient is experiencing acute decompensation or exacerbation of the underlying disease.
The next step, conducted by 2 researchers, was the process of developing, selecting and scoring items, using as reference the guideline for children with special health care needs of the NANEAS committee21 and the Questionnaire for Identifying Children with Chronic Conditions.22 The items referred to aspects directly associated with chronic courses of disease and medical complexity, and were assigned scores ranging from 0.5 to 4 points.
After their development, the items were rated by 5 evaluators, including paediatric hospitalists and paediatricians specialised in palliative care and the management of CCPs. Each of them rated the importance of the item for the calculation of content validity ratio (CVR) applying the Lawshe model modified by Tristán23 and the content validity index (CVI) of the scale. Items with a CVR of at least 0.58 were considered valid, and the validity of the whole final scale was determined based on a minimum CVI of 0.58.
In the second phase, confirmatory factor analysis was conducted to determine whether the scale fitted the observed data acceptably and whether any variables could be grouped. After the analysis, the CVI for the whole scale was calculated again. The internal consistency was assessed by means of the Cronbach alpha (0.7−0.9). The concordance and criterion validity were also analysed.
We assessed intra-rater agreement by means of test-retest analysis, applying the scale to each patient twice with 1 month between administrations. Inter-rater agreement was assessed by having the scale administered by a second rater, comparing the results with those obtained by the first one. We calculated the intraclass correlation coefficient for quantitative data (>0.7; 95% confidence interval [CI]) and the Cohen kappa coefficient for qualitative data (>0.7; P < .05).
Given the absence of validated methods to identify CCPs in our area with our working approach, the gold standard set for comparison was the classification as CCP or non-CCP by 2 evaluators specialised in the care of CCPs after reviewing the health records of the patient and taking into account the general aspects defined for the identification of CCPs in the methods section.
Each of these evaluators assessed 50% of the patients independently and, if there was any uncertainty about the classification of a patient, the classification was decided by consensus by both evaluators. The cut-off point for the definition of a patient as a CCP was established with a receiver operating characteristics (ROC) curve. After determining this threshold, we analysed the sensitivity and specificity of the scale.
Lastly, we confirmed that the patients classified as CCPs using the scale met the general criteria defined by the research group, and compared patients classified as CCPs and the diagnoses contemplated in version 2 of the Pediatric Complex Condition Classification system.16
The statistical analysis was performed with the software SPSS. We considered P values of less than .05 statistically significant.
ResultsThe scale was developed over a 1-year period, culminating with a version comprising 43 items and 11 sections (Table 1). The items that did not reach the minimum CVR of 0.58 were eliminated (7 items) or modified (12 items), and the resulting CVI for the overall test was 0.94.
Categories in the PedCom scale.
| 1) Specialty care |
| 2) Chronic medication |
| 3) Hospitalizations in the past 12 months |
| 4) Special dietary needs |
| 5) Need of respiratory care |
| 6) Psychomotor delay, mobility problems, functional limitations |
| 7) Vision problems and/or change in visual acuity with an impact on everyday activity |
| 8) Other devices or techniques |
| 9) Special treatment needs |
| 10) Special education needs |
| 11) Life expectancy <1 year or patient that could die within 12 months |
The pilot study included 180 patients: 90 patients who met the definition of CCP based on the gold standard, and 90 who did not, with a median age of 6 years and an interquartile range (IQR) of 8 years (2–10). Table 2 presents the specific clinics that managed the patients included in the study.
The confirmatory factor analysis led to the elimination of 1 item (peritoneal dialysis catheter) found to be redundant. After this correction, we obtained the final version comprising 42 items (see Appendix B, Supplementary data) grouped in 11 sections detailed in Table 1. The corresponding CVI for the overall test was 0.95.
The Cronbach alpha for the final version of the scale was 0.723.
The intraclass correlation coefficient obtained in the test-retest analysis with a 2-factor mixed effects model was 0.998 (95% CI, 0.997−0.998). As regards interrater agreement, we obtained an intraclass correlation coefficient of 0.996 (95% CI, 0.994−0.997) for the 2-way random effects model.
Based on the ROC curve analysis, we established a cut-off point for the definition of complex chronic patient of 6.5/27 points (sensitivity, 0.944; specificity, 0.78). Applying this cutoff, the Cohen kappa was 0.967 in the test-retest analysis (P < .01) and 0.946 in the analysis of interrater agreement (P < .01).
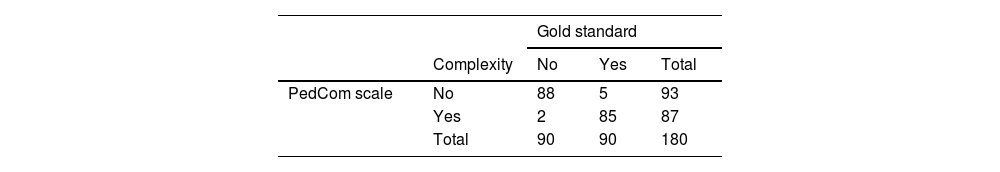
Table 3 presents the results of applying the developed scale compared to the results obtained with the gold standard.
Applying the established cutoff, the scale exhibited a sensitivity of 98% and a specificity of 94%, a positive predictive value of 95% and a negative predictive value of 98%.
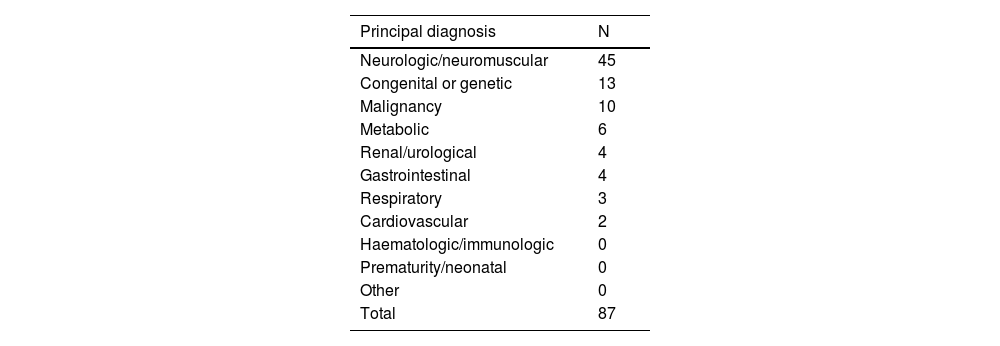
Applying the newly developed PedCom scale, 87 patients were classified as complex chronic patients. All of them met the general criteria established by the research group for the classification of a patient as a CCP. Ninety-two percent of the patients classified as CCP (80 patients) had at least 1 principal diagnosis included in the list of complex chronic conditions, and the rest met the criterion of having a CCC through a secondary diagnosis (intellectual disability, technological support or ventriculoperitoneal shunt). Thus, every patient classified as a CCP had a condition included in the CCC list.
Table 4 presents the classification of patients by category based on the system proposed by Feudtner et al.16
Distribution of patients classified as CCP with the PedCom scale based on the principal diagnosis.
| Principal diagnosis | N |
|---|---|
| Neurologic/neuromuscular | 45 |
| Congenital or genetic | 13 |
| Malignancy | 10 |
| Metabolic | 6 |
| Renal/urological | 4 |
| Gastrointestinal | 4 |
| Respiratory | 3 |
| Cardiovascular | 2 |
| Haematologic/immunologic | 0 |
| Prematurity/neonatal | 0 |
| Other | 0 |
| Total | 87 |
Our study offers a new approach, focused on the CCP as opposed to CCCs, to facilitate the identification of CCPs through an easy-to-use and intuitive tool, thus differentiating between having a CCC and being a CCP. This distinction helps narrow down the patients that may require special resources and follow-up by specialised complex chronic patient units.
From this perspective, classification as a CCP requires the presence of one or more CCCs in association with a variety of circumstances that result in long-term medical complexity, or, in other words, it is the accumulation of needs or functional limitations that lend complexity to a patient. Still, as is the case with CCCs, tools are needed to facilitate their identification that can be easily applied in the context of care delivery.
The PedCom scale is the first quantitative scale developed specifically for the identification of CCPs. It is easily applied and comprised of items that refer to essential aspects and needs of these patients. A need-based identification prevents the exclusion of patients who have yet to be diagnosed and the classification as CCP of patients with more than one chronic disease but without or with only mild functional impairment. The scale also assesses aspects like the need of special treatments or special education, which are not contemplated by other instruments.
The results of our study showed an adequate internal consistency in addition to adequate intra- and inter-rater agreement, with a good sensibility and specificity in the sample under study. However, a study in a larger sample is required for the definitive validation of the scale. There are also groups of patients with specific characteristics, such as those with malignancy, in whom the sensitivity and specificity of the scale should be tested specifically.
Since at present there is no widely used definition or tools to identify CCPs, the gold standard applied in the study was based on general criteria established by the research group and the classification performed by professionals involved in the management of these patients based on their health records. In consequence, the sensitivity and specificity found in our study may change if the scale is applied using a similar methodology to the one employed in our study to patients managed in other units. Therefore, we believe that performance of collaborative and multidisciplinary studies is essential to establish a standard definition for nationwide application, in addition to the development of standard protocols for the identification and follow-up of these patients.
In short, our study opens a new line of research to facilitate the identification of CCPs through an instrument that is easy to apply in everyday clinical practice; its ease of use allows application both by specialists and by clinicians at the primary care level, which would facilitate early referral of these patients to specialised units and their access to specific resources in the community. This would require performance of a study in the general population once the validation process is complete.
Lastly, this work set the foundations for a study by our group, already underway, to allow not only the identification of CCPs but also their classification based on the level of complexity.
FundingThe study received a grant from the Andalusian Pediatrics Society 2020 for a total of 5000€.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Dr. Antonio Urda Cardona for his support in the development of the PedCom and of a unit for the follow-up of complex chronic patients. We also thank the pediatric palliative care team of the Regional University Hospital that paved the way for improving the care of complex chronic pediatric patients.
Please cite this article as: Godoy-Molina E, Fernández-Ferrández T, Ruiz-Sánchez JM, Cordón-Martínez A, Pérez-Frías J, Navas-López VM, et al. Escala para la identificación del paciente pediátrico crónico complejo (EscalaPedCom). Estudio piloto. An Pediatr (Barc). 2022;97:155–160.